Abstract
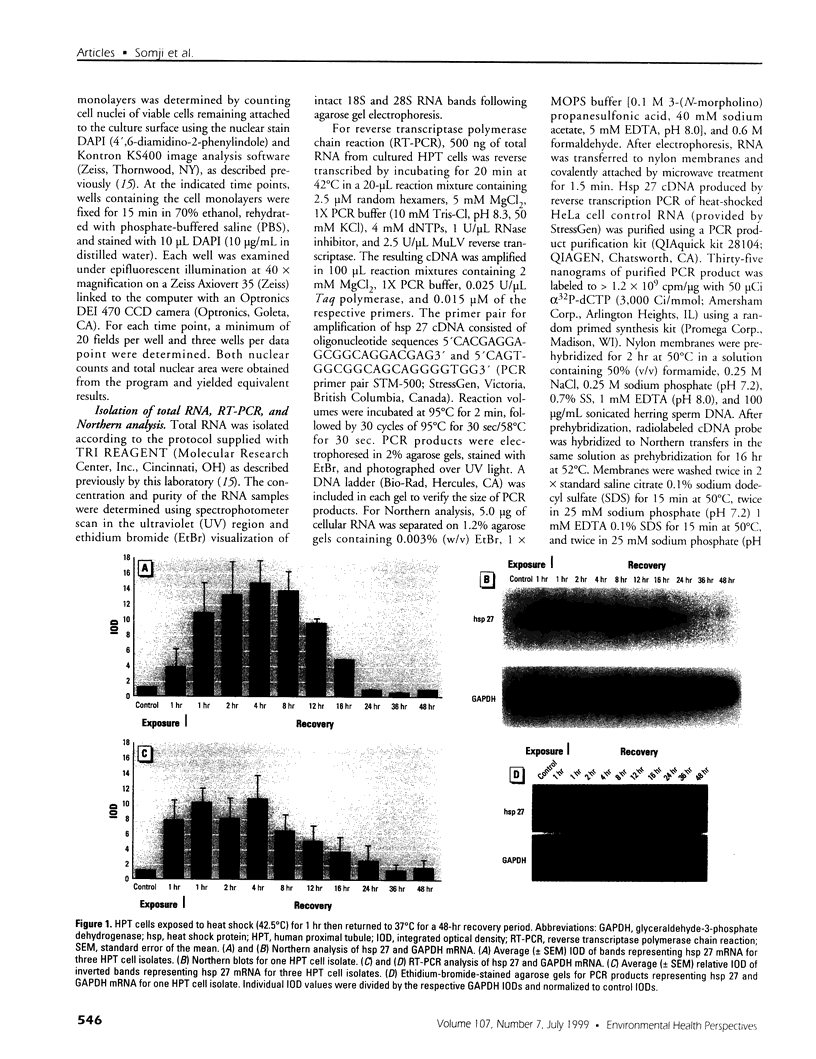
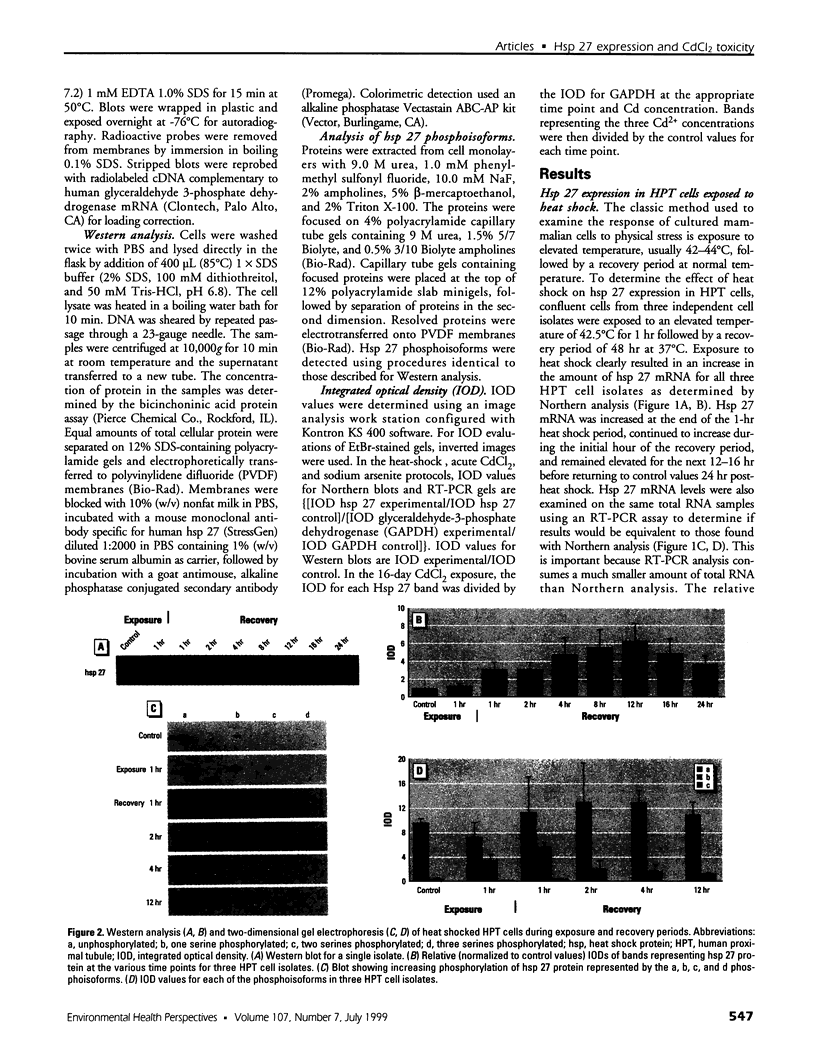
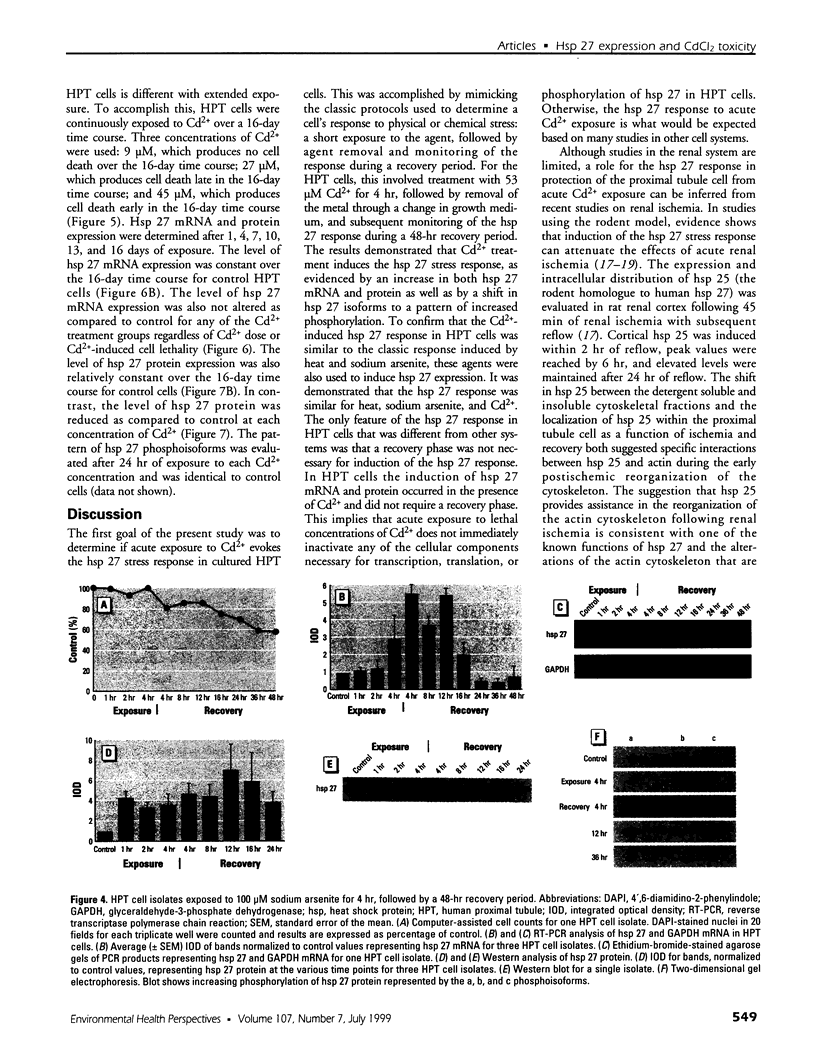
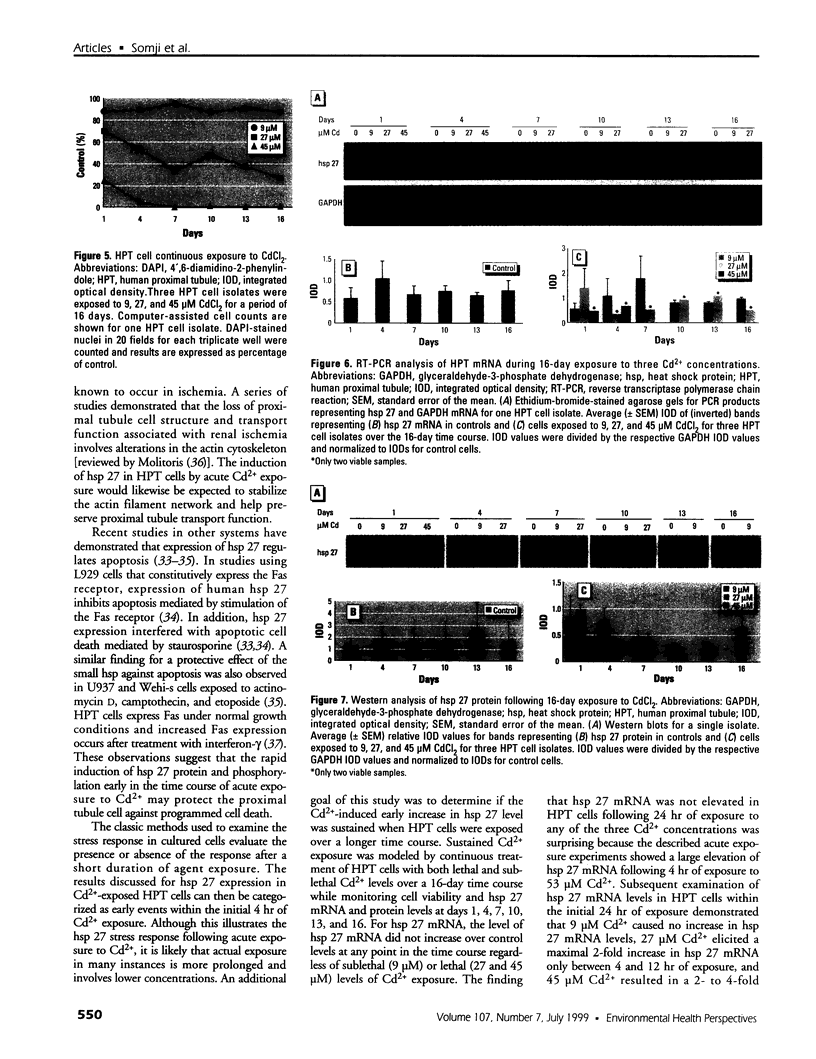
The expression of hsp 27 mRNA and protein was determined in cultured human proximal tubule (HPT) cells exposed to lethal and sublethal concentrations of Cd2+ under both acute and extended conditions. Initial procedures demonstrated that HPT cells display the classic stress response following physical and chemical stress. Heat stress (42.5 degrees C for 1 hr) caused an increase in both hsp 27 mRNA and protein as well as a shift in the protein to a more phosphorylated state. Results were similar when the cells were subjected to chemical stress (exposure to 100 microM sodium arsenite for 4 hr). Acute exposure to 53 microM CdCl2 for 4 hr also resulted in an increase in hsp 27 mRNA and protein and a shift to the more phosphorylated protein isoform. Extended Cd2+ exposure involved continuous treatment with Cd2+ at both lethal and sublethal levels over a 16-day time course. The results of this treatment showed that chronic exposure to Cd2+ failed to increase either hsp 27 mRNA or protein expression in HPT cells, even at lethal Cd2+ concentrations. In fact, hsp 27 protein levels decreased as compared to controls at both lethal and sub-lethal exposure to Cd2+. These findings imply that hsp 27 expression in human proximal tubule cells may have two distinct modes depending on the nature (acute vs. chronic) of the stress.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arrigo A. P. Small stress proteins: chaperones that act as regulators of intracellular redox state and programmed cell death. Biol Chem. 1998 Jan;379(1):19–26. [PubMed] [Google Scholar]
- Aufricht C., Ardito T., Thulin G., Kashgarian M., Siegel N. J., Van Why S. K. Heat-shock protein 25 induction and redistribution during actin reorganization after renal ischemia. Am J Physiol. 1998 Jan;274(1 Pt 2):F215–F222. doi: 10.1152/ajprenal.1998.274.1.F215. [DOI] [PubMed] [Google Scholar]
- Bernard A., Roels H., Hubermont G., Buchet J. P., Masson P. L., Lauwerys R. R. Characterization of the proteinuria in cadmium-exposed workers. Int Arch Occup Environ Health. 1976 Oct 21;38(1):19–30. doi: 10.1007/BF00378317. [DOI] [PubMed] [Google Scholar]
- Boonstra J. G., van der Woude F. J., Wever P. C., Laterveer J. C., Daha M. R., van Kooten C. Expression and function of Fas (CD95) on human renal tubular epithelial cells. J Am Soc Nephrol. 1997 Oct;8(10):1517–1524. doi: 10.1681/ASN.V8101517. [DOI] [PubMed] [Google Scholar]
- Bosco E., Porta N., Diezi J. Renal handling of cadmium: a study by tubular microinjections. Arch Toxicol Suppl. 1984;7:371–373. doi: 10.1007/978-3-642-69132-4_62. [DOI] [PubMed] [Google Scholar]
- Ciocca D. R., Oesterreich S., Chamness G. C., McGuire W. L., Fuqua S. A. Biological and clinical implications of heat shock protein 27,000 (Hsp27): a review. J Natl Cancer Inst. 1993 Oct 6;85(19):1558–1570. doi: 10.1093/jnci/85.19.1558. [DOI] [PubMed] [Google Scholar]
- Detrisac C. J., Sens M. A., Garvin A. J., Spicer S. S., Sens D. A. Tissue culture of human kidney epithelial cells of proximal tubule origin. Kidney Int. 1984 Feb;25(2):383–390. doi: 10.1038/ki.1984.28. [DOI] [PubMed] [Google Scholar]
- Garrett S. H., Somji S., Todd J. H., Sens D. A. Exposure of human proximal tubule cells to cd2+, zn2+, and Cu2+ induces metallothionein protein accumulation but not metallothionein isoform 2 mRNA. Environ Health Perspect. 1998 Sep;106(9):587–595. doi: 10.1289/ehp.98106587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrett S. H., Somji S., Todd J. H., Sens M. A., Sens D. A. Differential expression of human metallothionein isoform I mRNA in human proximal tubule cells exposed to metals. Environ Health Perspect. 1998 Dec;106(12):825–831. doi: 10.1289/ehp.98106825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgopoulos C., Welch W. J. Role of the major heat shock proteins as molecular chaperones. Annu Rev Cell Biol. 1993;9:601–634. doi: 10.1146/annurev.cb.09.110193.003125. [DOI] [PubMed] [Google Scholar]
- Hazen-Martin D. J., Sens D. A., Blackburn J. G., Flath M. C., Sens M. A. An electrophysiological freeze fracture assessment of cadmium nephrotoxicity in vitro. In Vitro Cell Dev Biol. 1989 Sep;25(9):791–799. doi: 10.1007/BF02623662. [DOI] [PubMed] [Google Scholar]
- Hazen-Martin D. J., Sens D. A., Blackburn J. G., Sens M. A. Cadmium nephrotoxicity in human proximal tubule cell cultures. In Vitro Cell Dev Biol. 1989 Sep;25(9):784–790. doi: 10.1007/BF02623661. [DOI] [PubMed] [Google Scholar]
- Hazen-Martin D. J., Todd J. H., Sens M. A., Khan W., Bylander J. E., Smyth B. J., Sens D. A. Electrical and freeze-fracture analysis of the effects of ionic cadmium on cell membranes of human proximal tubule cells. Environ Health Perspect. 1993 Nov;101(6):510–516. doi: 10.1289/ehp.93101510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoey J. G., Garrett S. H., Sens M. A., Todd J. H., Sens D. A. Expression of MT-3 mRNA in human kidney, proximal tubule cell cultures, and renal cell carcinoma. Toxicol Lett. 1997 Jul 21;92(2):149–160. doi: 10.1016/s0378-4274(97)00049-0. [DOI] [PubMed] [Google Scholar]
- Huot J., Houle F., Spitz D. R., Landry J. HSP27 phosphorylation-mediated resistance against actin fragmentation and cell death induced by oxidative stress. Cancer Res. 1996 Jan 15;56(2):273–279. [PubMed] [Google Scholar]
- Huot J., Roy G., Lambert H., Chrétien P., Landry J. Increased survival after treatments with anticancer agents of Chinese hamster cells expressing the human Mr 27,000 heat shock protein. Cancer Res. 1991 Oct 1;51(19):5245–5252. [PubMed] [Google Scholar]
- Kido T., Honda R., Tsuritani I., Yamaya H., Ishizaki M., Yamada Y., Nogawa K. Progress of renal dysfunction in inhabitants environmentally exposed to cadmium. Arch Environ Health. 1988 May-Jun;43(3):213–217. doi: 10.1080/00039896.1988.9934935. [DOI] [PubMed] [Google Scholar]
- Landry J., Chrétien P., Lambert H., Hickey E., Weber L. A. Heat shock resistance conferred by expression of the human HSP27 gene in rodent cells. J Cell Biol. 1989 Jul;109(1):7–15. doi: 10.1083/jcb.109.1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauwerys R. R., Buchet J. P., Roels H. A., Brouwers J., Stanescu D. Epidemiological survey of workers exposed to cadmium. Arch Environ Health. 1974 Mar;28(3):145–148. doi: 10.1080/00039896.1974.10666455. [DOI] [PubMed] [Google Scholar]
- Lavoie J. N., Gingras-Breton G., Tanguay R. M., Landry J. Induction of Chinese hamster HSP27 gene expression in mouse cells confers resistance to heat shock. HSP27 stabilization of the microfilament organization. J Biol Chem. 1993 Feb 15;268(5):3420–3429. [PubMed] [Google Scholar]
- Lavoie J. N., Hickey E., Weber L. A., Landry J. Modulation of actin microfilament dynamics and fluid phase pinocytosis by phosphorylation of heat shock protein 27. J Biol Chem. 1993 Nov 15;268(32):24210–24214. [PubMed] [Google Scholar]
- Lavoie J. N., Lambert H., Hickey E., Weber L. A., Landry J. Modulation of cellular thermoresistance and actin filament stability accompanies phosphorylation-induced changes in the oligomeric structure of heat shock protein 27. Mol Cell Biol. 1995 Jan;15(1):505–516. doi: 10.1128/mcb.15.1.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehlen P., Preville X., Chareyron P., Briolay J., Klemenz R., Arrigo A. P. Constitutive expression of human hsp27, Drosophila hsp27, or human alpha B-crystallin confers resistance to TNF- and oxidative stress-induced cytotoxicity in stably transfected murine L929 fibroblasts. J Immunol. 1995 Jan 1;154(1):363–374. [PubMed] [Google Scholar]
- Mehlen P., Schulze-Osthoff K., Arrigo A. P. Small stress proteins as novel regulators of apoptosis. Heat shock protein 27 blocks Fas/APO-1- and staurosporine-induced cell death. J Biol Chem. 1996 Jul 12;271(28):16510–16514. doi: 10.1074/jbc.271.28.16510. [DOI] [PubMed] [Google Scholar]
- Miron T., Vancompernolle K., Vandekerckhove J., Wilchek M., Geiger B. A 25-kD inhibitor of actin polymerization is a low molecular mass heat shock protein. J Cell Biol. 1991 Jul;114(2):255–261. doi: 10.1083/jcb.114.2.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PISCATOR M. Proteinuria in chronic cadmium poisoning. 2. The applicability of quantitative and qualitative methods of protein determination for the demonstration of cadmium proteinuria. Arch Environ Health. 1962 Oct;5:325–332. doi: 10.1080/00039896.1962.10663290. [DOI] [PubMed] [Google Scholar]
- Samali A., Cotter T. G. Heat shock proteins increase resistance to apoptosis. Exp Cell Res. 1996 Feb 25;223(1):163–170. doi: 10.1006/excr.1996.0070. [DOI] [PubMed] [Google Scholar]
- Schober A., Burger-Kentischer A., Müller E., Beck F. X. Effect of ischemia on localization of heat shock protein 25 in kidney. Kidney Int Suppl. 1998 Sep;67:S174–S176. doi: 10.1046/j.1523-1755.1998.06738.x. [DOI] [PubMed] [Google Scholar]
- Schober A., Müller E., Thurau K., Beck F. X. The response of heat shock proteins 25 and 72 to ischaemia in different kidney zones. Pflugers Arch. 1997 Jul;434(3):292–299. doi: 10.1007/s004240050399. [DOI] [PubMed] [Google Scholar]
- Welch W. J. Mammalian stress response: cell physiology, structure/function of stress proteins, and implications for medicine and disease. Physiol Rev. 1992 Oct;72(4):1063–1081. doi: 10.1152/physrev.1992.72.4.1063. [DOI] [PubMed] [Google Scholar]
- Wu W., Welsh M. J. Expression of the 25-kDa heat-shock protein (HSP27) correlates with resistance to the toxicity of cadmium chloride, mercuric chloride, cis-platinum(II)-diammine dichloride, or sodium arsenite in mouse embryonic stem cells transfected with sense or antisense HSP27 cDNA. Toxicol Appl Pharmacol. 1996 Nov;141(1):330–339. doi: 10.1006/taap.1996.0290. [DOI] [PubMed] [Google Scholar]