Abstract
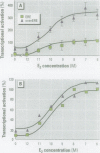
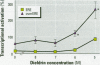
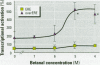
Xenoestrogens could be implicated in the decrease of male fertility and in the increased incidence of testicular and breast cancers in humans. To predict their deleterious effects, various in vivo or in vitro tests have been proposed to assay the xenoestrogenic activity. We have designed an assay for the detection of xenoestrogens based on a novel estrogen responsive unit formed by two overlapping estrogen response elements (overEREs). This construct is able to mediate a synergistic activation of transcription by 17ss-estradiol. We have used the overERE unit to assay the estrogenic activity of synthetic compounds, mostly organochlorine compounds. By using the overERE construct, we were able to detect the estrogenic activity of compounds at concentrations 10- to 100-fold lower than a single ERE (i.e., we detected the estrogenic effect of endosulfan at a concentration of 10(-5) M with ERE, whereas the overERE unit allowed us to detect a significant estrogenic activity of endosulfan at a lower concentration (10(-6) M). Some compounds did not exhibit any estrogenic activity when tested with a classical ERE, whereas they were potent xenoestrogens when the overERE was used (i.e., Betanal). The assays we have developed are very sensitive and can be performed quickly. Moreover, because the promoter that we used contains only an overlapping ERE as a regulatory unit, the interference of the tested molecules with other regulatory pathways can be avoided.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnold S. F., Robinson M. K., Notides A. C., Guillette L. J., Jr, McLachlan J. A. A yeast estrogen screen for examining the relative exposure of cells to natural and xenoestrogens. Environ Health Perspect. 1996 May;104(5):544–548. doi: 10.1289/ehp.96104544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auger J., Kunstmann J. M., Czyglik F., Jouannet P. Decline in semen quality among fertile men in Paris during the past 20 years. N Engl J Med. 1995 Feb 2;332(5):281–285. doi: 10.1056/NEJM199502023320501. [DOI] [PubMed] [Google Scholar]
- Beato M. Gene regulation by steroid hormones. Cell. 1989 Feb 10;56(3):335–344. doi: 10.1016/0092-8674(89)90237-7. [DOI] [PubMed] [Google Scholar]
- Beato M., Herrlich P., Schütz G. Steroid hormone receptors: many actors in search of a plot. Cell. 1995 Dec 15;83(6):851–857. doi: 10.1016/0092-8674(95)90201-5. [DOI] [PubMed] [Google Scholar]
- Brandt M. E., Vickery L. E. Cooperativity and dimerization of recombinant human estrogen receptor hormone-binding domain. J Biol Chem. 1997 Feb 21;272(8):4843–4849. doi: 10.1074/jbc.272.8.4843. [DOI] [PubMed] [Google Scholar]
- Carlsen E., Giwercman A., Keiding N., Skakkebaek N. E. Evidence for decreasing quality of semen during past 50 years. BMJ. 1992 Sep 12;305(6854):609–613. doi: 10.1136/bmj.305.6854.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chilvers C., Pike M. C., Forman D., Fogelman K., Wadsworth M. E. Apparent doubling of frequency of undescended testis in England and Wales in 1962-81. Lancet. 1984 Aug 11;2(8398):330–332. doi: 10.1016/s0140-6736(84)92697-7. [DOI] [PubMed] [Google Scholar]
- Czeizel A., Tóth J., Czvenits E. Increased birth prevalence of isolated hypospadias in Hungary. Acta Paediatr Hung. 1986;27(4):329–337. [PubMed] [Google Scholar]
- Das S. K., Tan J., Johnson D. C., Dey S. K. Differential spatiotemporal regulation of lactoferrin and progesterone receptor genes in the mouse uterus by primary estrogen, catechol estrogen, and xenoestrogen. Endocrinology. 1998 Jun;139(6):2905–2915. doi: 10.1210/endo.139.6.6051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis D. L., Bradlow H. L., Wolff M., Woodruff T., Hoel D. G., Anton-Culver H. Medical hypothesis: xenoestrogens as preventable causes of breast cancer. Environ Health Perspect. 1993 Oct;101(5):372–377. doi: 10.1289/ehp.93101372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean D. M., Sanders M. M. Ten years after: reclassification of steroid-responsive genes. Mol Endocrinol. 1996 Dec;10(12):1489–1495. doi: 10.1210/mend.10.12.8961259. [DOI] [PubMed] [Google Scholar]
- Evans R. M. The steroid and thyroid hormone receptor superfamily. Science. 1988 May 13;240(4854):889–895. doi: 10.1126/science.3283939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forman D., Møller H. Testicular cancer. Cancer Surv. 1994;19-20:323–341. [PubMed] [Google Scholar]
- Guillette L. J., Jr, Pickford D. B., Crain D. A., Rooney A. A., Percival H. F. Reduction in penis size and plasma testosterone concentrations in juvenile alligators living in a contaminated environment. Gen Comp Endocrinol. 1996 Jan;101(1):32–42. doi: 10.1006/gcen.1996.0005. [DOI] [PubMed] [Google Scholar]
- Klein-Hitpass L., Ryffel G. U., Heitlinger E., Cato A. C. A 13 bp palindrome is a functional estrogen responsive element and interacts specifically with estrogen receptor. Nucleic Acids Res. 1988 Jan 25;16(2):647–663. doi: 10.1093/nar/16.2.647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knowles B. B., Howe C. C., Aden D. P. Human hepatocellular carcinoma cell lines secrete the major plasma proteins and hepatitis B surface antigen. Science. 1980 Jul 25;209(4455):497–499. doi: 10.1126/science.6248960. [DOI] [PubMed] [Google Scholar]
- Kumar V., Chambon P. The estrogen receptor binds tightly to its responsive element as a ligand-induced homodimer. Cell. 1988 Oct 7;55(1):145–156. doi: 10.1016/0092-8674(88)90017-7. [DOI] [PubMed] [Google Scholar]
- Maitre J. L., Mercier L., Dolo L., Valotaire Y. Caractérisation de récepteurs spécifiques à l'oestradiol, induction de la vitellogénine et de son mRNA dans le foie de truite arc-en-ci el (Salmo gairdnerii). Biochimie. 1985 Feb;67(2):215–225. doi: 10.1016/s0300-9084(85)80050-x. [DOI] [PubMed] [Google Scholar]
- Mangelsdorf D. J., Evans R. M. The RXR heterodimers and orphan receptors. Cell. 1995 Dec 15;83(6):841–850. doi: 10.1016/0092-8674(95)90200-7. [DOI] [PubMed] [Google Scholar]
- Mangelsdorf D. J., Thummel C., Beato M., Herrlich P., Schütz G., Umesono K., Blumberg B., Kastner P., Mark M., Chambon P. The nuclear receptor superfamily: the second decade. Cell. 1995 Dec 15;83(6):835–839. doi: 10.1016/0092-8674(95)90199-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massaad C., Coumoul X., Sabbah M., Garlatti M., Redeuilh G., Barouki R. Properties of overlapping EREs: synergistic activation of transcription and cooperative binding of ER. Biochemistry. 1998 Apr 28;37(17):6023–6032. doi: 10.1021/bi972445e. [DOI] [PubMed] [Google Scholar]
- Massaad C., Lombès M., Aggerbeck M., Rafestin-Oblin M. E., Barouki R. Cell-specific, promoter-dependent mineralocorticoid agonist activity of spironolactone. Mol Pharmacol. 1997 Feb;51(2):285–292. doi: 10.1124/mol.51.2.285. [DOI] [PubMed] [Google Scholar]
- Nelson C. M., Bunge R. G. Semen analysis: evidence for changing parameters of male fertility potential. Fertil Steril. 1974 Jun;25(6):503–507. doi: 10.1016/s0015-0282(16)40454-1. [DOI] [PubMed] [Google Scholar]
- Newbold R. R., Bullock B. C., McLachlan J. A. Adenocarcinoma of the rete testis. Diethylstilbestrol-induced lesions of the mouse rete testis. Am J Pathol. 1986 Dec;125(3):625–628. [PMC free article] [PubMed] [Google Scholar]
- Newbold R. R., Bullock B. C., McLachlan J. A. Lesions of the rete testis in mice exposed prenatally to diethylstilbestrol. Cancer Res. 1985 Oct;45(10):5145–5150. [PubMed] [Google Scholar]
- Sonnenschein C., Soto A. M., Fernandez M. F., Olea N., Olea-Serrano M. F., Ruiz-Lopez M. D. Development of a marker of estrogenic exposure in human serum. Clin Chem. 1995 Dec;41(12 Pt 2):1888–1895. [PubMed] [Google Scholar]
- Sonnenschein C., Szelei J., Nye T. L., Soto A. M. Control of cell proliferation of human breast MCF7 cells; serum and estrogen resistant variants. Oncol Res. 1994;6(8):373–381. [PubMed] [Google Scholar]
- Soto A. M., Chung K. L., Sonnenschein C. The pesticides endosulfan, toxaphene, and dieldrin have estrogenic effects on human estrogen-sensitive cells. Environ Health Perspect. 1994 Apr;102(4):380–383. doi: 10.1289/ehp.94102380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swan S. H., Elkin E. P., Fenster L. Have sperm densities declined? A reanalysis of global trend data. Environ Health Perspect. 1997 Nov;105(11):1228–1232. doi: 10.1289/ehp.971051228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweet R. A., Schrott H. G., Kurland R., Culp O. S. Study of the incidence of hypospadias in Rochester, Minnesota, 1940-1970, and a case-control comparison of possible etiologic factors. Mayo Clin Proc. 1974 Jan;49(1):52–58. [PubMed] [Google Scholar]
- Toppari J., Larsen J. C., Christiansen P., Giwercman A., Grandjean P., Guillette L. J., Jr, Jégou B., Jensen T. K., Jouannet P., Keiding N. Male reproductive health and environmental xenoestrogens. Environ Health Perspect. 1996 Aug;104 (Suppl 4):741–803. doi: 10.1289/ehp.96104s4741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umesono K., Giguere V., Glass C. K., Rosenfeld M. G., Evans R. M. Retinoic acid and thyroid hormone induce gene expression through a common responsive element. Nature. 1988 Nov 17;336(6196):262–265. doi: 10.1038/336262a0. [DOI] [PubMed] [Google Scholar]
- de Wet J. R., Wood K. V., DeLuca M., Helinski D. R., Subramani S. Firefly luciferase gene: structure and expression in mammalian cells. Mol Cell Biol. 1987 Feb;7(2):725–737. doi: 10.1128/mcb.7.2.725. [DOI] [PMC free article] [PubMed] [Google Scholar]