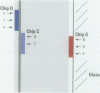
Abstract
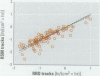
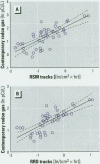
We performed both a laboratory and a field intercomparison of two novel glass-based retrospective radon detectors previously used in major radon case-control studies performed in Missouri and Iowa. The new detectors estimate retrospective residential radon exposure from the accumulation of a long-lived radon decay product, (210)Pb, in glass. The detectors use track registration material in direct contact with glass surfaces to measure the alpha-emission of a (210)Pb-decay product, (210)Po. The detector's track density generation rate (tracks per square centimeter per hour) is proportional to the surface alpha-activity. In the absence of other strong sources of alpha-emission in the glass, the implanted surface alpha-activity should be proportional to the accumulated (210)Po, and hence to the cumulative radon gas exposure. The goals of the intercomparison were to a) perform collocated measurements using two different glass-based retrospective radon detectors in a controlled laboratory environment to compare their relative response to implanted polonium in the absence of environmental variation, b) perform collocated measurements using two different retrospective radon progeny detectors in a variety of residential settings to compare their detection of glass-implanted polonium activities, and c) examine the correlation between track density rates and contemporary radon gas concentrations. The laboratory results suggested that the materials and methods used by the studies produced similar track densities in detectors exposed to the same implanted (210)Po activity. The field phase of the intercomparison found excellent agreement between the track density rates for the two types of retrospective detectors. The correlation between the track density rates and direct contemporary radon concentration measurements was relatively high, considering that no adjustments were performed to account for either the residential depositional environment or glass surface type. Preliminary comparisons of the models used to translate track rate densities to average long-term radon concentrations differ between the two studies. Further calibration of the retrospective detectors' models for interpretation of track rate density may allow the pooling of studies that use glass-based retrospective radon detectors to determine historic residential radon exposures.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alavanja M. C., Brownson R. C., Lubin J. H., Berger E., Chang J., Boice J. D., Jr Residential radon exposure and lung cancer among nonsmoking women. J Natl Cancer Inst. 1994 Dec 21;86(24):1829–1837. doi: 10.1093/jnci/86.24.1829. [DOI] [PubMed] [Google Scholar]
- Alavanja M. C., Lubin J. H., Mahaffey J. A., Brownson R. C. Residential radon exposure and risk of lung cancer in Missouri. Am J Public Health. 1999 Jul;89(7):1042–1048. doi: 10.2105/ajph.89.7.1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auvinen A., Mäkeläinen I., Hakama M., Castrén O., Pukkala E., Reisbacka H., Rytömaa T. Indoor radon exposure and risk of lung cancer: a nested case-control study in Finland. J Natl Cancer Inst. 1996 Jul 17;88(14):966–972. doi: 10.1093/jnci/88.14.966. [DOI] [PubMed] [Google Scholar]
- Baumert E., Schlesier M., Wolff-Vorbeck G., Peter H. H. Veränderungen von Lymphozyten-subpopulationen bei variablem Immundefektsyndrom. Immun Infekt. 1992 Jul;20(3):73–75. [PubMed] [Google Scholar]
- Blot W. J., Xu Z. Y., Boice J. D., Jr, Zhao D. Z., Stone B. J., Sun J., Jing L. B., Fraumeni J. F., Jr Indoor radon and lung cancer in China. J Natl Cancer Inst. 1990 Jun 20;82(12):1025–1030. doi: 10.1093/jnci/82.12.1025. [DOI] [PubMed] [Google Scholar]
- Darby S., Whitley E., Silcocks P., Thakrar B., Green M., Lomas P., Miles J., Reeves G., Fearn T., Doll R. Risk of lung cancer associated with residential radon exposure in south-west England: a case-control study. Br J Cancer. 1998 Aug;78(3):394–408. doi: 10.1038/bjc.1998.506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field R. W., Steck D. J., Lynch C. F., Brus C. P., Neuberger J. S., Kross B. C. Residential radon-222 exposure and lung cancer: exposure assessment methodology. J Expo Anal Environ Epidemiol. 1996 Apr-Jun;6(2):181–195. [PubMed] [Google Scholar]
- Hess C. T., Fleischer R. L., Turner L. G. Field and laboratory tests of etched track detectors for 222Rn: summer-vs-winter variations and tightness effects in Maine houses. Health Phys. 1985 Jul;49(1):65–79. doi: 10.1097/00004032-198507000-00006. [DOI] [PubMed] [Google Scholar]
- Lively R. S., Ney E. P. Surface radioactivity resulting from the deposition of 222Rn daughter products. Health Phys. 1987 Apr;52(4):411–415. doi: 10.1097/00004032-198704000-00001. [DOI] [PubMed] [Google Scholar]
- Lively R. S., Steck D. J. Long-term radon concentrations estimated from 210Po embedded in glass. Health Phys. 1993 May;64(5):485–490. doi: 10.1097/00004032-199305000-00005. [DOI] [PubMed] [Google Scholar]
- Lubin J. H., Samet J. M., Weinberg C. Design issues in epidemiologic studies of indoor exposure to Rn and risk of lung cancer. Health Phys. 1990 Dec;59(6):807–817. doi: 10.1097/00004032-199012000-00004. [DOI] [PubMed] [Google Scholar]
- Létourneau E. G., Krewski D., Choi N. W., Goddard M. J., McGregor R. G., Zielinski J. M., Du J. Case-control study of residential radon and lung cancer in Winnipeg, Manitoba, Canada. Am J Epidemiol. 1994 Aug 15;140(4):310–322. doi: 10.1093/oxfordjournals.aje.a117253. [DOI] [PubMed] [Google Scholar]
- Mahaffey J. A., Parkhurst M. A., James A. C., Cross F. T., Alavanja M. C., Boice J. D., Ezrine S., Henderson P., Brownson R. C. Estimating past exposure to indoor radon from household glass. Health Phys. 1993 Apr;64(4):381–391. doi: 10.1097/00004032-199304000-00005. [DOI] [PubMed] [Google Scholar]
- Pershagen G., Akerblom G., Axelson O., Clavensjö B., Damber L., Desai G., Enflo A., Lagarde F., Mellander H., Svartengren M. Residential radon exposure and lung cancer in Sweden. N Engl J Med. 1994 Jan 20;330(3):159–164. doi: 10.1056/NEJM199401203300302. [DOI] [PubMed] [Google Scholar]
- Pershagen G., Liang Z. H., Hrubec Z., Svensson C., Boice J. D., Jr Residential radon exposure and lung cancer in Swedish women. Health Phys. 1992 Aug;63(2):179–186. doi: 10.1097/00004032-199208000-00004. [DOI] [PubMed] [Google Scholar]
- Ruosteenoja E., Mäkeläinen I., Rytömaa T., Hakulinen T., Hakama M. Radon and lung cancer in Finland. Health Phys. 1996 Aug;71(2):185–189. doi: 10.1097/00004032-199608000-00009. [DOI] [PubMed] [Google Scholar]
- Samuelsson C. Retrospective determination of radon in houses. Nature. 1988 Jul 28;334(6180):338–340. doi: 10.1038/334338a0. [DOI] [PubMed] [Google Scholar]
- Schoenberg J. B., Klotz J. B., Wilcox H. B., Nicholls G. P., Gil-del-Real M. T., Stemhagen A., Mason T. J. Case-control study of residential radon and lung cancer among New Jersey women. Cancer Res. 1990 Oct 15;50(20):6520–6524. [PubMed] [Google Scholar]
- Steck D. J. Spatial and temporal indoor radon variations. Health Phys. 1992 Apr;62(4):351–355. doi: 10.1097/00004032-199204000-00009. [DOI] [PubMed] [Google Scholar]