Abstract
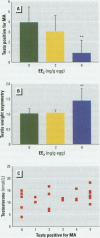
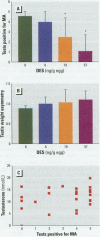
Chemicals having a capacity to disturb the endocrine system have attracted considerable interest during recent years. There is a shortage of well-characterized in vivo tests with which to study such disturbances in different classes of vertebrates. In the present study, test end points related to reproduction in the Japanese quail were used to examine the estrogenic activity of chemicals. The synthetic estrogens ethinylestradiol (EE(2)) and diethylstilbestrol (DES), used as model compounds, were injected into the yolk of embryonated eggs. After the birds had been raised to sexual maturity, we examined sexual behavior, plasma testosterone concentrations, and testis morphology in adult males. The lowest doses resulting in a significantly depressed male sexual behavior were 6 ng/g egg for EE(2) and 19 ng/g egg for DES. Testis weight asymmetry was increased at 6 ng EE(2)/g egg, but DES had no effect at any treatment level. The area of the androgen-dependent cloacal gland was significantly reduced at 57 ng DES/g egg. No effects on plasma testosterone concentration or body weight following exposure to EE(2) or DES were observed at any dose level. Depressed male sexual behavior was the most sensitive of the end points studied, and we suggest that this ecologically relevant end point be included in avian in vivo testing for neuroendocrine disruptors.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adkins E. K., Adler N. T. Hormonal control of behavior in the Japanese quail. J Comp Physiol Psychol. 1972 Oct;81(1):27–36. doi: 10.1037/h0033315. [DOI] [PubMed] [Google Scholar]
- Adkins E. K., Boop J. J., Koutnik D. L., Morris J. B., Pniewski E. E. Further evidence that androgen aromatization is essential for the activation of copulation in male quail. Physiol Behav. 1980 Mar;24(3):441–446. doi: 10.1016/0031-9384(80)90233-4. [DOI] [PubMed] [Google Scholar]
- Adkins E. K. Effect of embryonic treatment with estradiol or testosterone on sexual differentiation of the quail brain. Critical period and dose-response relationships. Neuroendocrinology. 1979;29(3):178–185. doi: 10.1159/000122920. [DOI] [PubMed] [Google Scholar]
- Adkins E. K. Embryonic exposure to an antiestrogen masculinizes behavior of female quail. Physiol Behav. 1976 Aug;17(2):357–359. doi: 10.1016/0031-9384(76)90088-3. [DOI] [PubMed] [Google Scholar]
- Adkins E. K. Hormonal basis of sexual differentiation in the Japanese quail. J Comp Physiol Psychol. 1975 Mar;89(1):61–71. doi: 10.1037/h0076406. [DOI] [PubMed] [Google Scholar]
- Alexandre C., Balthazart J. Effects of metabolism inhibitors, antiestrogens and antiandrogens on the androgen and estrogen induced sexual behavior in Japanese quail. Physiol Behav. 1986 Oct;38(4):581–591. doi: 10.1016/0031-9384(86)90429-4. [DOI] [PubMed] [Google Scholar]
- Aste N., Panzica G. C., Viglietti-Panzica C., Balthazart J. Effects of in ovo estradiol benzoate treatments on sexual behavior and size of neurons in the sexually dimorphic medial preoptic nucleus of Japanese quail. Brain Res Bull. 1991 Nov;27(5):713–720. doi: 10.1016/0361-9230(91)90051-k. [DOI] [PubMed] [Google Scholar]
- Balthazart J., Castagna C., Ball G. F. Differential effects of D1 and D2 dopamine-receptor agonists and antagonists on appetitive and consummatory aspects of male sexual behavior in Japanese quail. Physiol Behav. 1997 Sep;62(3):571–580. doi: 10.1016/s0031-9384(97)00163-7. [DOI] [PubMed] [Google Scholar]
- Balthazart J., De Clerck A., Foidart A. Behavioral demasculinization of female quail is induced by estrogens: studies with the new aromatase inhibitor, R76713. Horm Behav. 1992 Jun;26(2):179–203. doi: 10.1016/0018-506x(92)90041-s. [DOI] [PubMed] [Google Scholar]
- Balthazart J., Surlemont C. Androgen and estrogen action in the preoptic area and activation of copulatory behavior in quail. Physiol Behav. 1990 Nov;48(5):599–609. doi: 10.1016/0031-9384(90)90198-d. [DOI] [PubMed] [Google Scholar]
- Balthazart J., Tlemçani O., Ball G. F. Do sex differences in the brain explain sex differences in the hormonal induction of reproductive behavior? What 25 years of research on the Japanese quail tells us. Horm Behav. 1996 Dec;30(4):627–661. doi: 10.1006/hbeh.1996.0066. [DOI] [PubMed] [Google Scholar]
- Berg C., Halldin K., Fridolfsson A. K., Brandt I., Brunström B. The avian egg as a test system for endocrine disrupters: effects of diethylstilbestrol and ethynylestradiol on sex organ development. Sci Total Environ. 1999 Aug 15;233(1-3):57–66. doi: 10.1016/s0048-9697(99)00179-5. [DOI] [PubMed] [Google Scholar]
- Bolger R., Wiese T. E., Ervin K., Nestich S., Checovich W. Rapid screening of environmental chemicals for estrogen receptor binding capacity. Environ Health Perspect. 1998 Sep;106(9):551–557. doi: 10.1289/ehp.98106551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunström B., Darnerud P. O. Toxicity and distribution in chick embryos of 3,3',4,4'-tetrachlorobiphenyl injected into the eggs. Toxicology. 1983 Jun;27(2):103–110. doi: 10.1016/0300-483x(83)90015-x. [DOI] [PubMed] [Google Scholar]
- Bryan T. E., Gildersleeve R. P., Wiard R. P. Exposure of Japanese quail embryos to o,p'-DDT has long-term effects on reproductive behaviors, hematology, and feather morphology. Teratology. 1989 Jun;39(6):525–535. doi: 10.1002/tera.1420390603. [DOI] [PubMed] [Google Scholar]
- Castagna C., Ball G. F., Balthazart J. Effects of dopamine agonists on appetitive and consummatory male sexual behavior in Japanese quail. Pharmacol Biochem Behav. 1997 Oct;58(2):403–414. doi: 10.1016/s0091-3057(97)00243-8. [DOI] [PubMed] [Google Scholar]
- Crain D. A., Guillette L. J., Jr, Rooney A. A., Pickford D. B. Alterations in steroidogenesis in alligators (Alligator mississippiensis) exposed naturally and experimentally to environmental contaminants. Environ Health Perspect. 1997 May;105(5):528–533. doi: 10.1289/ehp.97105528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crews D., Bergeron J. M., McLachlan J. A. The role of estrogen in turtle sex determination and the effect of PCBs. Environ Health Perspect. 1995 Oct;103 (Suppl 7):73–77. doi: 10.1289/ehp.95103s773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DORFMAN R. I., DORFMAN A. S. Estrogen assays using the rat uterus. Endocrinology. 1954 Jul;55(1):65–69. doi: 10.1210/endo-55-1-65. [DOI] [PubMed] [Google Scholar]
- Facemire C. F., Gross T. S., Guillette L. J., Jr Reproductive impairment in the Florida panther: nature or nurture? Environ Health Perspect. 1995 May;103 (Suppl 4):79–86. doi: 10.1289/ehp.103-1519283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foidart A., Harada N., Balthazart J. Effects of steroidal and non steroidal aromatase inhibitors on sexual behavior and aromatase-immunoreactive cells and fibers in the quail brain. Brain Res. 1994 Sep 19;657(1-2):105–123. doi: 10.1016/0006-8993(94)90958-x. [DOI] [PubMed] [Google Scholar]
- Fry D. M. Reproductive effects in birds exposed to pesticides and industrial chemicals. Environ Health Perspect. 1995 Oct;103 (Suppl 7):165–171. doi: 10.1289/ehp.95103s7165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fry D. M., Toone C. K. DDT-induced feminization of gull embryos. Science. 1981 Aug 21;213(4510):922–924. doi: 10.1126/science.7256288. [DOI] [PubMed] [Google Scholar]
- Gaido K. W., Leonard L. S., Lovell S., Gould J. C., Babaï D., Portier C. J., McDonnell D. P. Evaluation of chemicals with endocrine modulating activity in a yeast-based steroid hormone receptor gene transcription assay. Toxicol Appl Pharmacol. 1997 Mar;143(1):205–212. doi: 10.1006/taap.1996.8069. [DOI] [PubMed] [Google Scholar]
- Gildersleeve R. P., Tilson H. A., Mitchell C. L. Injection of diethylstilbestrol on the first day of incubation affects morphology of sex glands and reproductive behavior of Japanese quail. Teratology. 1985 Feb;31(1):101–109. doi: 10.1002/tera.1420310112. [DOI] [PubMed] [Google Scholar]
- Gray L. E., Jr, Ostby J. Effects of pesticides and toxic substances on behavioral and morphological reproductive development: endocrine versus nonendocrine mechanisms. Toxicol Ind Health. 1998 Jan-Apr;14(1-2):159–184. doi: 10.1177/074823379801400111. [DOI] [PubMed] [Google Scholar]
- Guillette L. J., Jr, Gross T. S., Masson G. R., Matter J. M., Percival H. F., Woodward A. R. Developmental abnormalities of the gonad and abnormal sex hormone concentrations in juvenile alligators from contaminated and control lakes in Florida. Environ Health Perspect. 1994 Aug;102(8):680–688. doi: 10.1289/ehp.94102680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hikim A. P., Amador A. G., Klemcke H. G., Bartke A., Russell L. D. Correlative morphology and endocrinology of Sertoli cells in hamster testes in active and inactive states of spermatogenesis. Endocrinology. 1989 Oct;125(4):1829–1843. doi: 10.1210/endo-125-4-1829. [DOI] [PubMed] [Google Scholar]
- Hutchison R. E. Hormonal differentiation of sexual behavior in Japanese quail. Horm Behav. 1978 Dec;11(3):363–387. doi: 10.1016/0018-506x(78)90038-7. [DOI] [PubMed] [Google Scholar]
- MacLellan K. N., Bird D. M., Fry D. M., Cowles J. L. Reproductive and morphological effects of o,p'-dicofol on two generations of captive American kestrels. Arch Environ Contam Toxicol. 1996 Mar;30(3):364–372. doi: 10.1007/BF00212295. [DOI] [PubMed] [Google Scholar]
- Nagel S. C., vom Saal F. S., Thayer K. A., Dhar M. G., Boechler M., Welshons W. V. Relative binding affinity-serum modified access (RBA-SMA) assay predicts the relative in vivo bioactivity of the xenoestrogens bisphenol A and octylphenol. Environ Health Perspect. 1997 Jan;105(1):70–76. doi: 10.1289/ehp.9710570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ottinger M. A., Follett B. K. Circulating levels of luteinizing hormone and androgen in mature male Japanese quail over 24 hours. Poult Sci. 1987 Aug;66(8):1411–1413. doi: 10.3382/ps.0661411. [DOI] [PubMed] [Google Scholar]
- Panzica G. C., Castagna C., Viglietti-Panzica C., Russo C., Tlemçani O., Balthazart J. Organizational effects of estrogens on brain vasotocin and sexual behavior in quail. J Neurobiol. 1998 Dec;37(4):684–699. doi: 10.1002/(sici)1097-4695(199812)37:4<684::aid-neu15>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- Panzica G. C., Viglietti-Panzica C., Balthazart J. The sexually dimorphic medial preoptic nucleus of quail: a key brain area mediating steroid action on male sexual behavior. Front Neuroendocrinol. 1996 Jan;17(1):51–125. doi: 10.1006/frne.1996.0002. [DOI] [PubMed] [Google Scholar]
- Peterson R. E., Theobald H. M., Kimmel G. L. Developmental and reproductive toxicity of dioxins and related compounds: cross-species comparisons. Crit Rev Toxicol. 1993;23(3):283–335. doi: 10.3109/10408449309105013. [DOI] [PubMed] [Google Scholar]
- RUBIN B. L., DORFMAN A. S., BLACK L., DORFMAN R. I. Bioassay of estrogens using the mouse uterine response. Endocrinology. 1951 Oct;49(4):429–439. doi: 10.1210/endo-49-4-429. [DOI] [PubMed] [Google Scholar]
- Scheib D., Guichard A., Mignot T. M., Cedard L. Steroidogenesis by gonads of normal and of diethylstilbestrol-treated quail embryos: radioimmunoassays on organ cultures. Gen Comp Endocrinol. 1981 Apr;43(4):519–526. doi: 10.1016/0016-6480(81)90236-7. [DOI] [PubMed] [Google Scholar]
- Scheib D., Reyss-Brion M. Feminization of the quail by early diethylstilbestrol treatment: histoenzymological investigations on steroid dehydrogenases in the gonads. Arch Anat Microsc Morphol Exp. 1979;68(2):85–98. [PubMed] [Google Scholar]
- Schumacher M., Hendrick J. C., Balthazart J. Sexual differentiation in quail: critical period and hormonal specificity. Horm Behav. 1989 Mar;23(1):130–149. doi: 10.1016/0018-506x(89)90080-9. [DOI] [PubMed] [Google Scholar]
- Soto A. M., Justicia H., Wray J. W., Sonnenschein C. p-Nonyl-phenol: an estrogenic xenobiotic released from "modified" polystyrene. Environ Health Perspect. 1991 May;92:167–173. doi: 10.1289/ehp.9192167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soto A. M., Sonnenschein C., Chung K. L., Fernandez M. F., Olea N., Serrano F. O. The E-SCREEN assay as a tool to identify estrogens: an update on estrogenic environmental pollutants. Environ Health Perspect. 1995 Oct;103 (Suppl 7):113–122. doi: 10.1289/ehp.95103s7113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinmetz R., Mitchner N. A., Grant A., Allen D. L., Bigsby R. M., Ben-Jonathan N. The xenoestrogen bisphenol A induces growth, differentiation, and c-fos gene expression in the female reproductive tract. Endocrinology. 1998 Jun;139(6):2741–2747. doi: 10.1210/endo.139.6.6027. [DOI] [PubMed] [Google Scholar]
- White R., Jobling S., Hoare S. A., Sumpter J. P., Parker M. G. Environmentally persistent alkylphenolic compounds are estrogenic. Endocrinology. 1994 Jul;135(1):175–182. doi: 10.1210/endo.135.1.8013351. [DOI] [PubMed] [Google Scholar]
- Whitsett J. M., Irvin E. W., Edens F. W., Thaxton J. P. Demasculinization of male japanese quail by prenatal estrogen treatment. Horm Behav. 1977 Apr;8(2):254–263. doi: 10.1016/0018-506x(77)90042-3. [DOI] [PubMed] [Google Scholar]
- Wilson J. A., Glick B. Ontogeny of mating behavior in the chicken. Am J Physiol. 1970 Apr;218(4):951–955. doi: 10.1152/ajplegacy.1970.218.4.951. [DOI] [PubMed] [Google Scholar]
- vom Saal F. S., Timms B. G., Montano M. M., Palanza P., Thayer K. A., Nagel S. C., Dhar M. D., Ganjam V. K., Parmigiani S., Welshons W. V. Prostate enlargement in mice due to fetal exposure to low doses of estradiol or diethylstilbestrol and opposite effects at high doses. Proc Natl Acad Sci U S A. 1997 Mar 4;94(5):2056–2061. doi: 10.1073/pnas.94.5.2056. [DOI] [PMC free article] [PubMed] [Google Scholar]