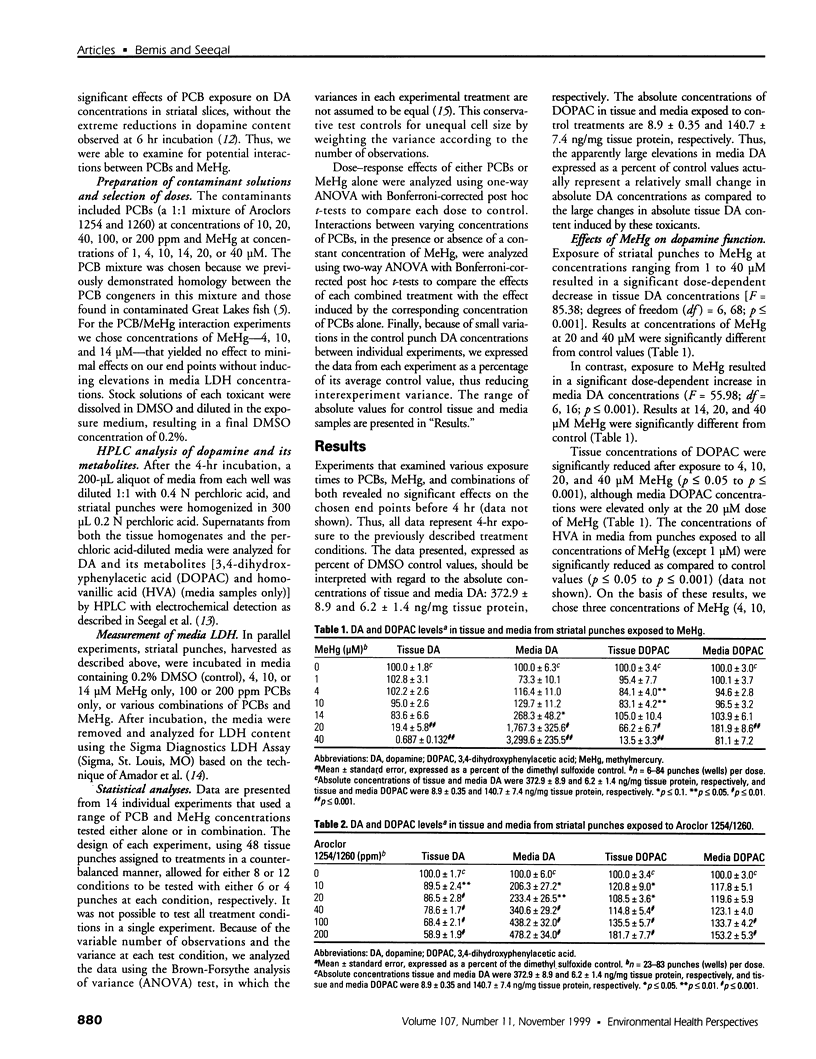
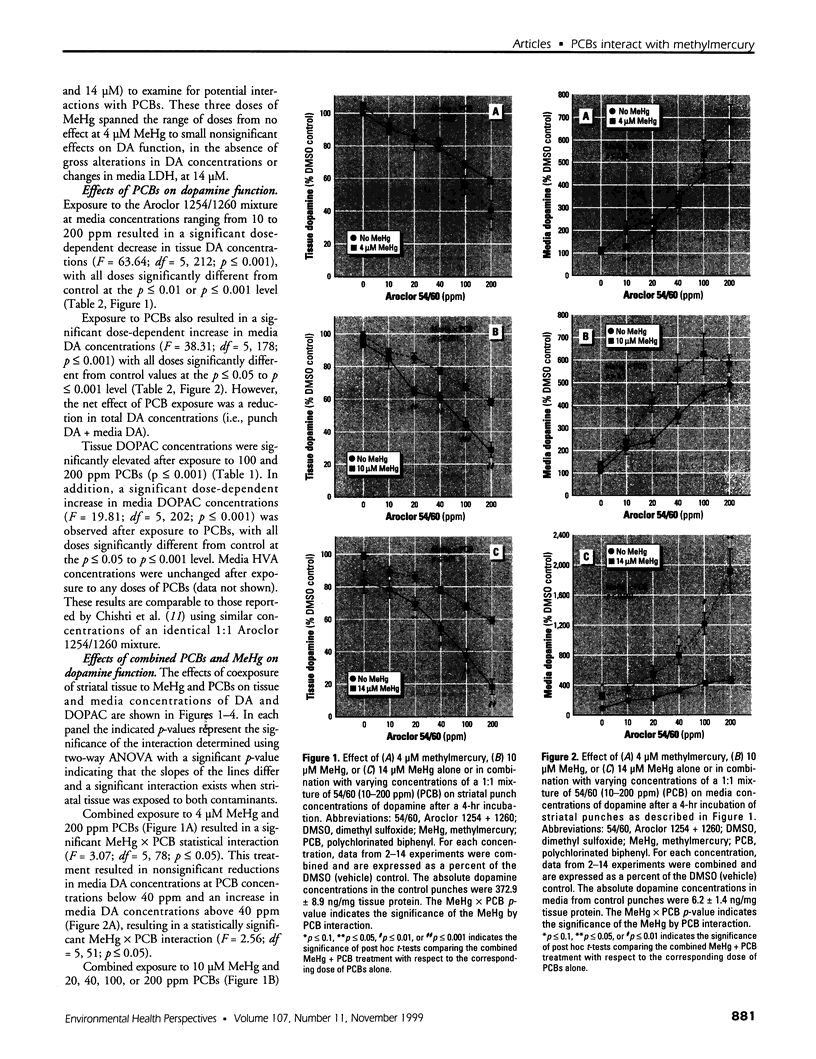
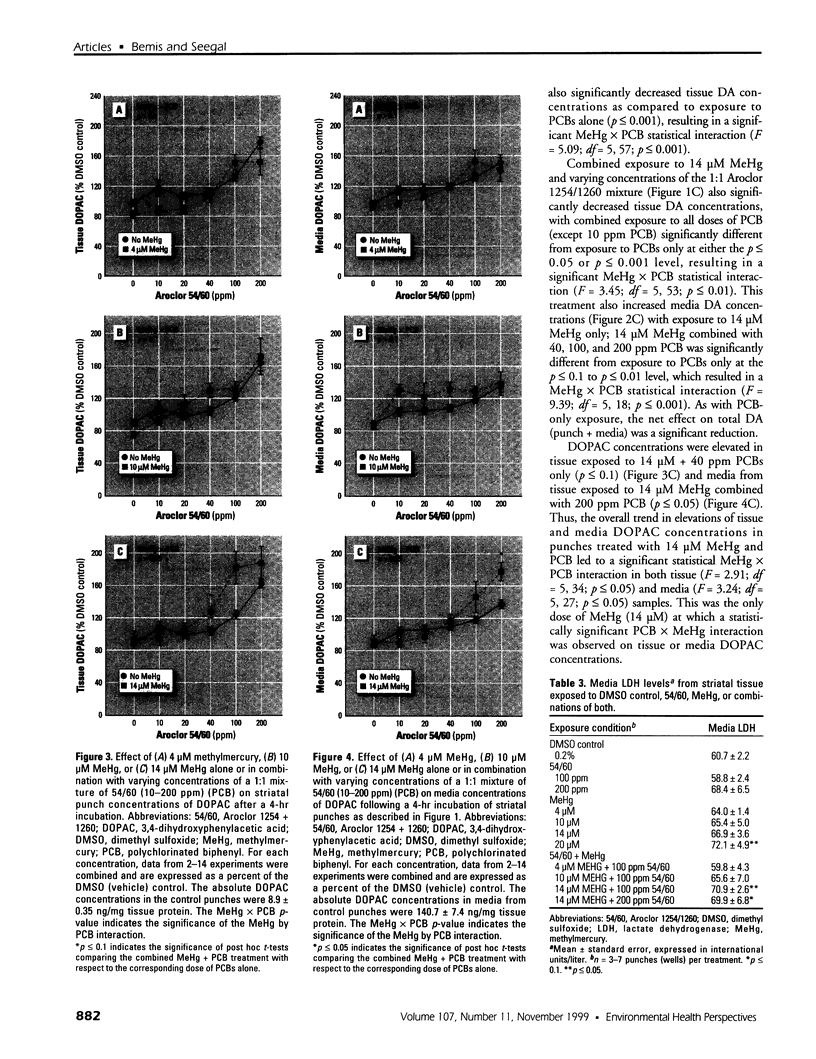
Abstract
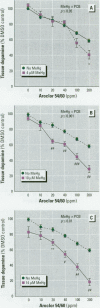
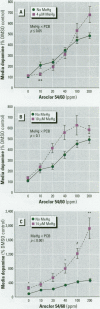
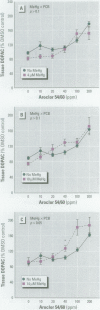
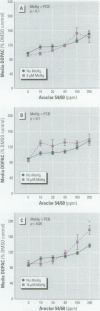
Consumption of contaminated Great Lakes fish by pregnant women is associated with decreased birth weight and deficits in cognitive function in their infants and children. These fish contain many known and suspected anthropogenic neurotoxicants, making it difficult to determine which contaminant(s) are responsible for the observed deficits. We have undertaken a series of experiments to determine the relevant toxicants by comparing the neurotoxic effects of two of these contaminants--polychlorinated biphenyls (PCBs) and methylmercury (MeHg)--both of which are recognized neurotoxicants. Striatal punches obtained from adult rat brain were exposed to PCBs only, MeHg only, or the two in combination, and tissue and media concentrations of dopamine (DA) and its metabolites were determined by high performance liquid chromatography. Exposure to PCBs only reduced tissue DA and elevated media DA in a dose-dependent fashion. Exposure to MeHg only did not significantly affect either measure. However, when striatal punches were simultaneously exposed to PCBs and MeHg, there were significantly greater decreases in tissue DA concentrations and elevations in media DA than those caused by PCBs only, in the absence of changes in media lactate dehydrogenase concentrations. Elevations in both tissue and media 3, 4-dihydroxyphenylacetic acid concentrations were also observed. We suggest that the significant interactions between these two toxicants may be due to a common site of action (i.e., toxicant-induced increases in intracellular calcium and changes in second messenger systems) that influences DA function. The synergism between these contaminants suggests that future revisions of fish-consumption guidelines should consider contaminant interactions.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AMADOR E., DORFMAN L. E., WACKER W. E. SERUM LACTIC DEHYDROGENASE ACTIVITY: AN ANALYTICAL ASSESSMENT OF CURRENT ASSAYS. Clin Chem. 1963 Aug;12:391–399. [PubMed] [Google Scholar]
- Chishti M. A., Fisher J. P., Seegal R. F. Aroclors 1254 and 1260 reduce dopamine concentrations in rat striatal slices. Neurotoxicology. 1996 Fall-Winter;17(3-4):653–660. [PubMed] [Google Scholar]
- Choksi N. Y., Kodavanti P. R., Tilson H. A., Booth R. G. Effects of polychlorinated biphenyls (PCBs) on brain tyrosine hydroxylase activity and dopamine synthesis in rats. Fundam Appl Toxicol. 1997 Sep;39(1):76–80. doi: 10.1006/faat.1997.2351. [DOI] [PubMed] [Google Scholar]
- Copeland B. J., Vogelsberg V., Neff N. H., Hadjiconstantinou M. Protein kinase C activators decrease dopamine uptake into striatal synaptosomes. J Pharmacol Exp Ther. 1996 Jun;277(3):1527–1532. [PubMed] [Google Scholar]
- Crespi D., Mennini T., Gobbi M. Carrier-dependent and Ca(2+)-dependent 5-HT and dopamine release induced by (+)-amphetamine, 3,4-methylendioxymethamphetamine, p-chloroamphetamine and (+)-fenfluramine. Br J Pharmacol. 1997 Aug;121(8):1735–1743. doi: 10.1038/sj.bjp.0701325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faro L. R., Durán R., do Nascimento J. L., Alfonso M., Picanço-Diniz C. W. Effects of methyl mercury on the in vivo release of dopamine and its acidic metabolites DOPAC and HVA from striatum of rats. Ecotoxicol Environ Saf. 1997 Nov;38(2):95–98. doi: 10.1006/eesa.1997.1567. [DOI] [PubMed] [Google Scholar]
- Feeley M. M., Jordan S. A. Dietary and tissue residue analysis and contaminant intake estimations in rats consuming diets composed of Great Lakes salmon: a multigeneration study. Regul Toxicol Pharmacol. 1998 Feb;27(1 Pt 2):S8–S17. doi: 10.1006/rtph.1997.1187. [DOI] [PubMed] [Google Scholar]
- Hanneman W. H., Legare M. E., Barhoumi R., Burghardt R. C., Safe S., Tiffany-Castiglioni E. Stimulation of calcium uptake in cultured rat hippocampal neurons by 2,3,7,8-tetrachlorodibenzo-p-dioxin. Toxicology. 1996 Aug 1;112(1):19–28. doi: 10.1016/0300-483x(96)03346-x. [DOI] [PubMed] [Google Scholar]
- Jacobson J. L., Jacobson S. W., Humphrey H. E. Effects of exposure to PCBs and related compounds on growth and activity in children. Neurotoxicol Teratol. 1990 Jul-Aug;12(4):319–326. doi: 10.1016/0892-0362(90)90050-m. [DOI] [PubMed] [Google Scholar]
- Jacobson J. L., Jacobson S. W., Humphrey H. E. Effects of in utero exposure to polychlorinated biphenyls and related contaminants on cognitive functioning in young children. J Pediatr. 1990 Jan;116(1):38–45. doi: 10.1016/s0022-3476(05)81642-7. [DOI] [PubMed] [Google Scholar]
- Jacobson J. L., Jacobson S. W. Intellectual impairment in children exposed to polychlorinated biphenyls in utero. N Engl J Med. 1996 Sep 12;335(11):783–789. doi: 10.1056/NEJM199609123351104. [DOI] [PubMed] [Google Scholar]
- Kalisch B. E., Racz W. J. The effects of methylmercury on endogenous dopamine efflux from mouse striatal slices. Toxicol Lett. 1996 Dec;89(1):43–49. doi: 10.1016/s0378-4274(96)03787-3. [DOI] [PubMed] [Google Scholar]
- Kennedy M. B. Regulation of neuronal function by calcium. Trends Neurosci. 1989 Nov;12(11):417–420. doi: 10.1016/0166-2236(89)90089-1. [DOI] [PubMed] [Google Scholar]
- Kodavanti P. R., Shin D. S., Tilson H. A., Harry G. J. Comparative effects of two polychlorinated biphenyl congeners on calcium homeostasis in rat cerebellar granule cells. Toxicol Appl Pharmacol. 1993 Nov;123(1):97–106. doi: 10.1006/taap.1993.1226. [DOI] [PubMed] [Google Scholar]
- Korzeniewski C., Callewaert D. M. An enzyme-release assay for natural cytotoxicity. J Immunol Methods. 1983 Nov 25;64(3):313–320. doi: 10.1016/0022-1759(83)90438-6. [DOI] [PubMed] [Google Scholar]
- Kumer S. C., Vrana K. E. Intricate regulation of tyrosine hydroxylase activity and gene expression. J Neurochem. 1996 Aug;67(2):443–462. doi: 10.1046/j.1471-4159.1996.67020443.x. [DOI] [PubMed] [Google Scholar]
- Lonart G., Zigmond M. J. Incubation of tissue slices in the absence of Ca2+ and Mg2+ can cause nonspecific damage. J Neurochem. 1991 Apr;56(4):1445–1448. doi: 10.1111/j.1471-4159.1991.tb11445.x. [DOI] [PubMed] [Google Scholar]
- Marty M. S., Atchison W. D. Pathways mediating Ca2+ entry in rat cerebellar granule cells following in vitro exposure to methyl mercury. Toxicol Appl Pharmacol. 1997 Dec;147(2):319–330. doi: 10.1006/taap.1997.8262. [DOI] [PubMed] [Google Scholar]
- Messeri M. D., Bickmeyer U., Weinsberg F., Wiegand H. Congener specific effects by polychlorinated biphenyls on catecholamine content and release in chromaffin cells. Arch Toxicol. 1997;71(7):416–421. doi: 10.1007/s002040050405. [DOI] [PubMed] [Google Scholar]
- Minnema D. J., Cooper G. P., Greenland R. D. Effects of methylmercury on neurotransmitter release from rat brain synaptosomes. Toxicol Appl Pharmacol. 1989 Jul;99(3):510–521. doi: 10.1016/0041-008x(89)90158-0. [DOI] [PubMed] [Google Scholar]
- Palarea M. D., Castro R., Arévalo R. M., Rodríguez-Díaz M. Papel de los receptores presinápticos en el control de la síntesis de dopamina en el complejo estriado y en el núcleo accumbens. Rev Esp Fisiol. 1986 Mar;42(1):71–75. [PubMed] [Google Scholar]
- Sarafian T. A. Methyl mercury increases intracellular Ca2+ and inositol phosphate levels in cultured cerebellar granule neurons. J Neurochem. 1993 Aug;61(2):648–657. doi: 10.1111/j.1471-4159.1993.tb02169.x. [DOI] [PubMed] [Google Scholar]
- Seegal R. F., Brosch K. O., Bush B. High-performance liquid chromatography of biogenic amines and metabolites in brain, cerebrospinal fluid, urine and plasma. J Chromatogr. 1986 Apr 25;377:131–144. doi: 10.1016/s0378-4347(00)80768-9. [DOI] [PubMed] [Google Scholar]
- Seegal R. F. Epidemiological and laboratory evidence of PCB-induced neurotoxicity. Crit Rev Toxicol. 1996 Nov;26(6):709–737. doi: 10.3109/10408449609037481. [DOI] [PubMed] [Google Scholar]
- Seegal R. F., Pappas B. A., Park G. A. Neurochemical effects of consumption of Great Lakes salmon by rats. Regul Toxicol Pharmacol. 1998 Feb;27(1 Pt 2):S68–S75. doi: 10.1006/rtph.1997.1192. [DOI] [PubMed] [Google Scholar]
- Shain W., Bush B., Seegal R. Neurotoxicity of polychlorinated biphenyls: structure-activity relationship of individual congeners. Toxicol Appl Pharmacol. 1991 Oct;111(1):33–42. doi: 10.1016/0041-008x(91)90131-w. [DOI] [PubMed] [Google Scholar]
- Tilson H. A., Kodavanti P. R. The neurotoxicity of polychlorinated biphenyls. Neurotoxicology. 1998 Aug-Oct;19(4-5):517–525. [PubMed] [Google Scholar]
- Vaughan R. A., Huff R. A., Uhl G. R., Kuhar M. J. Protein kinase C-mediated phosphorylation and functional regulation of dopamine transporters in striatal synaptosomes. J Biol Chem. 1997 Jun 13;272(24):15541–15546. doi: 10.1074/jbc.272.24.15541. [DOI] [PubMed] [Google Scholar]
- Weihe P., Grandjean P., Debes F., White R. Health implications for Faroe islanders of heavy metals and PCBs from pilot whales. Sci Total Environ. 1996 Jul 16;186(1-2):141–148. doi: 10.1016/0048-9697(96)05094-2. [DOI] [PubMed] [Google Scholar]
- Westerink B. H., Van Putten F. M. Simultaneous determination of the rates of synthesis and metabolism of dopamine in various areas of the rat brain: application to the effects of (+)-amphetamine. Eur J Pharmacol. 1987 Jan 6;133(1):103–110. doi: 10.1016/0014-2999(87)90211-1. [DOI] [PubMed] [Google Scholar]
- Wolf M. E., Galloway M. P., Roth R. H. Regulation of dopamine synthesis in the medial prefrontal cortex: studies in brain slices. J Pharmacol Exp Ther. 1986 Mar;236(3):699–707. [PubMed] [Google Scholar]
- Wolf M. E., Roth R. H. Autoreceptor regulation of dopamine synthesis. Ann N Y Acad Sci. 1990;604:323–343. doi: 10.1111/j.1749-6632.1990.tb32003.x. [DOI] [PubMed] [Google Scholar]
- Yang X. F., Lee B. L., New A. L., Ong H. Y., Ma L., Zhang Q., Ong C. N. Urinary homovanillic acid and vanillylmandelic acid in workers exposed to carbon disulfide. Am J Ind Med. 1996 Mar;29(3):269–274. doi: 10.1002/(SICI)1097-0274(199603)29:3<269::AID-AJIM6>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]