Abstract
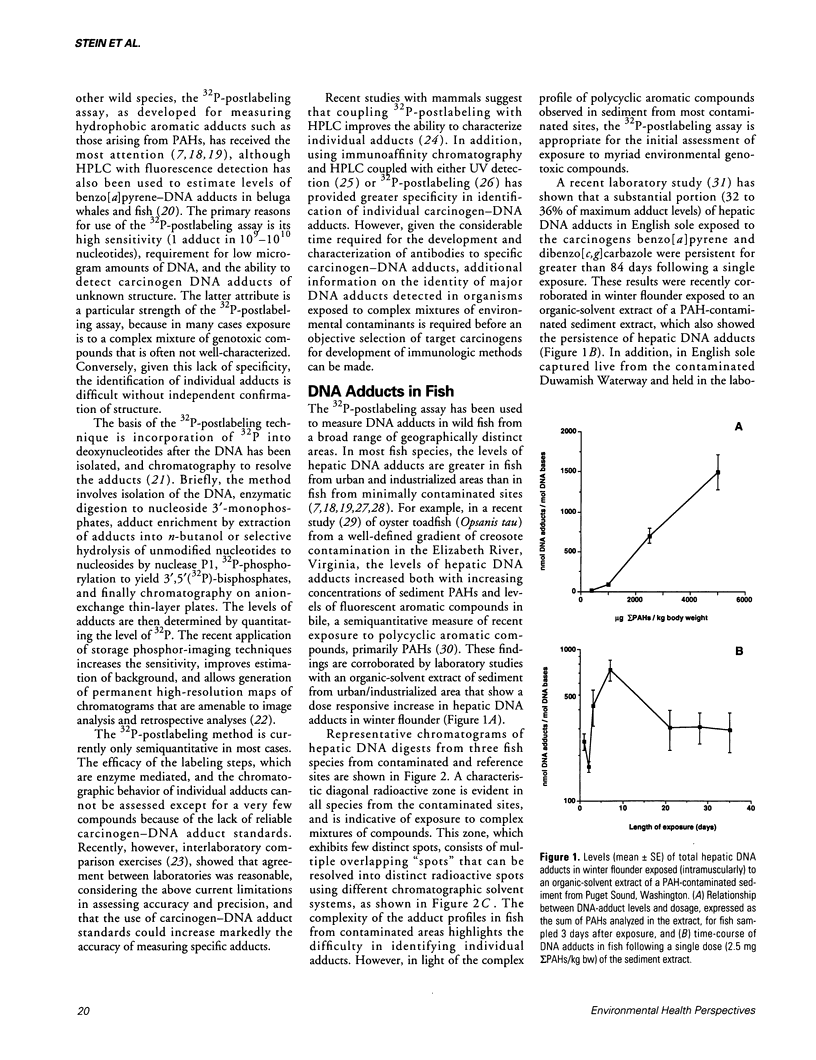
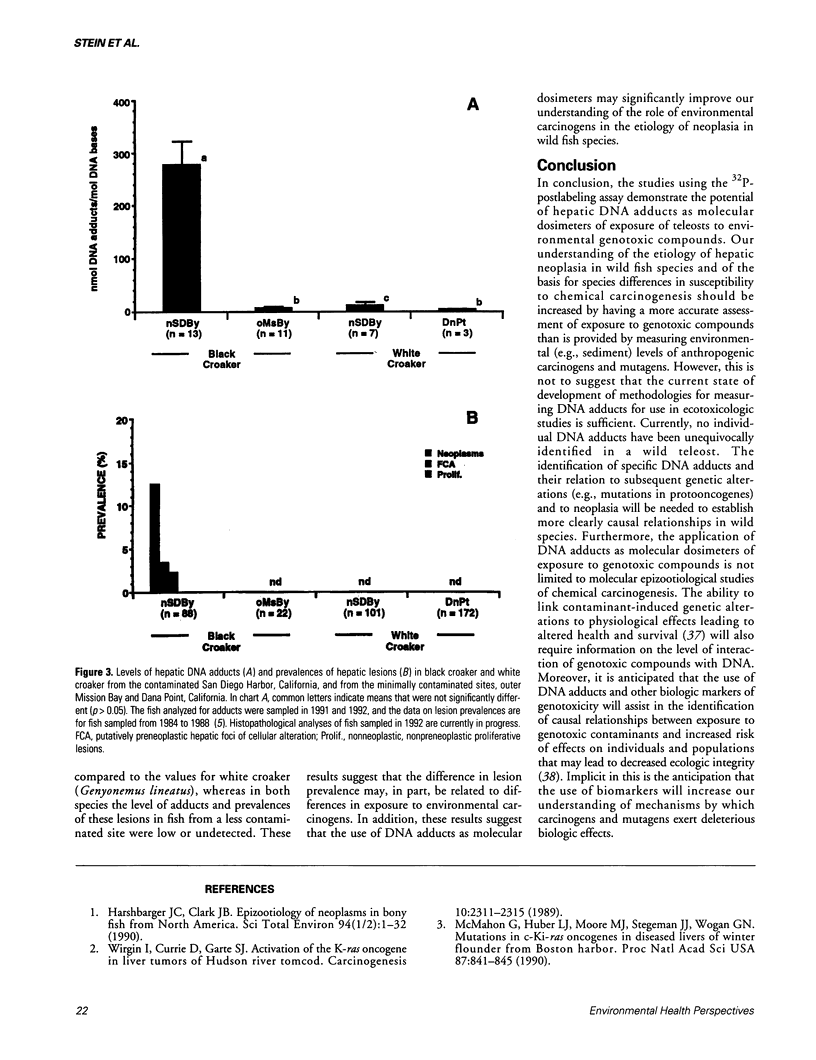
The recent development of techniques to measure levels of carcinogens covalently bound to DNA provides the opportunity to use DNA adducts as molecular dosimeters of exposure to environmental carcinogens and mutagens. This is especially important because epizootiologic studies have shown a positive association between environmental carcinogens, such as polycyclic aromatic hydrocarbons, and increased prevalence of neoplasms and related lesions, primarily in liver, of benthic fish species from a wide range of urban and industrialized areas. In studies with wild fish and mammalian species the 32P-postlabeling assay, as developed for aromatic compounds, has been used most extensively because of its high sensitivity and ability to detect structurally uncharacterized adducts. The results to date of field and laboratory studies show that hepatic DNA adducts detected in fish are associated with increased exposure to environmental polycyclic aromatic compounds in the preponderance of species examined, whereas in the limited studies with wild mammals, such a relationship is equivocal at present. The findings with fish suggest that DNA adducts, as measured by 32P-postlabeling, have the potential to be effective molecular dosimeters of exposure to environmental carcinogenic aromatic compounds and thereby may lead to an improved understanding of the etiology of neoplasia in wild teleosts.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Dunn B. P., Black J. J., Maccubbin A. 32P-postlabeling analysis of aromatic DNA adducts in fish from polluted areas. Cancer Res. 1987 Dec 15;47(24 Pt 1):6543–6548. [PubMed] [Google Scholar]
- Fong A. T., Dashwood R. H., Cheng R., Mathews C., Ford B., Hendricks J. D., Bailey G. S. Carcinogenicity, metabolism and Ki-ras proto-oncogene activation by 7,12-dimethylbenz[a]anthracene in rainbow trout embryos. Carcinogenesis. 1993 Apr;14(4):629–635. doi: 10.1093/carcin/14.4.629. [DOI] [PubMed] [Google Scholar]
- Groopman J. D., Hasler J. A., Trudel L. J., Pikul A., Donahue P. R., Wogan G. N. Molecular dosimetry in rat urine of aflatoxin-N7-guanine and other aflatoxin metabolites by multiple monoclonal antibody affinity chromatography and immunoaffinity/high performance liquid chromatography. Cancer Res. 1992 Jan 15;52(2):267–274. [PubMed] [Google Scholar]
- Harshbarger J. C., Clark J. B. Epizootiology of neoplasms in bony fish of North America. Sci Total Environ. 1990 May 1;94(1-2):1–32. doi: 10.1016/0048-9697(90)90362-x. [DOI] [PubMed] [Google Scholar]
- Hawkins W. E., Walker W. W., Overstreet R. M., Lytle J. S., Lytle T. F. Carcinogenic effects of some polycyclic aromatic hydrocarbons on the Japanese medaka and guppy in waterborne exposures. Sci Total Environ. 1990 May 1;94(1-2):155–167. doi: 10.1016/0048-9697(90)90370-a. [DOI] [PubMed] [Google Scholar]
- Hendricks J. D., Meyers T. R., Shelton D. W., Casteel J. L., Bailey G. S. Hepatocarcinogenicity of benzo[a]pyrene to rainbow trout by dietary exposure and intraperitoneal injection. J Natl Cancer Inst. 1985 Apr;74(4):839–851. [PubMed] [Google Scholar]
- Landahl J. T., McCain B. B., Myers M. S., Rhodes L. D., Brown D. W. Consistent associations between hepatic lesions in English sole (Parophrys vetulus) and polycyclic aromatic hydrocarbons in bottom sediment. Environ Health Perspect. 1990 Nov;89:195–203. doi: 10.1289/ehp.9089195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu T. Y., Cheng S. L., Ueng T. H., Ueng Y. F., Chi C. W. Comparative analysis of aromatic DNA adducts in fish from polluted and unpolluted areas by the 32P-postlabeling analysis. Bull Environ Contam Toxicol. 1991 Nov;47(5):783–789. doi: 10.1007/BF01701150. [DOI] [PubMed] [Google Scholar]
- McMahon G., Huber L. J., Moore M. J., Stegeman J. J., Wogan G. N. Mutations in c-Ki-ras oncogenes in diseased livers of winter flounder from Boston Harbor. Proc Natl Acad Sci U S A. 1990 Jan;87(2):841–845. doi: 10.1073/pnas.87.2.841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Möller L., Zeisig M., Vodicka P. Optimization of an HPLC method for analyses of 32P-postlabeled DNA adducts. Carcinogenesis. 1993 Jul;14(7):1343–1348. doi: 10.1093/carcin/14.7.1343. [DOI] [PubMed] [Google Scholar]
- Randerath K., Reddy M. V., Disher R. M. Age- and tissue-related DNA modifications in untreated rats: detection by 32P-postlabeling assay and possible significance for spontaneous tumor induction and aging. Carcinogenesis. 1986 Sep;7(9):1615–1617. doi: 10.1093/carcin/7.9.1615. [DOI] [PubMed] [Google Scholar]
- Reichert W. L., Stein J. E., French B., Goodwin P., Varanasi U. Storage phosphor imaging technique for detection and quantitation of DNA adducts measured by the 32P-postlabeling assay. Carcinogenesis. 1992 Aug;13(8):1475–1479. doi: 10.1093/carcin/13.8.1475. [DOI] [PubMed] [Google Scholar]
- Savela K., Hemminki K., Hewer A., Phillips D. H., Putman K. L., Randerath K. Interlaboratory comparison of the 32P-postlabelling assay for aromatic DNA adducts in white blood cells of iron foundry workers. Mutat Res. 1989 Dec;224(4):485–492. doi: 10.1016/0165-1218(89)90074-8. [DOI] [PubMed] [Google Scholar]
- Schultz M. E., Schultz R. J. Induction of hepatic tumors with 7,12-dimethylbenz[a]anthracene in two species of viviparous fishes (Genus poeciliopsis). Environ Res. 1982 Apr;27(2):337–351. doi: 10.1016/0013-9351(82)90089-5. [DOI] [PubMed] [Google Scholar]
- Shields P. G., Bowman E. D., Harrington A. M., Doan V. T., Weston A. Polycyclic aromatic hydrocarbon-DNA adducts in human lung and cancer susceptibility genes. Cancer Res. 1993 Aug 1;53(15):3486–3492. [PubMed] [Google Scholar]
- Stein J. E., Reichert W. L., French B., Varanasi U. 32P-postlabeling analysis of DNA adduct formation and persistence in English sole (Pleuronectes vetulus) exposed to benzo[a]pyrene and 7H-dibenzo[c,g]carbazole. Chem Biol Interact. 1993 Jul;88(1):55–69. doi: 10.1016/0009-2797(93)90084-c. [DOI] [PubMed] [Google Scholar]
- Varanasi U., Reichert W. L., Stein J. E. 32P-postlabeling analysis of DNA adducts in liver of wild English sole (Parophrys vetulus) and winter flounder (Pseudopleuronectes americanus). Cancer Res. 1989 Mar 1;49(5):1171–1177. [PubMed] [Google Scholar]
- Wirgin I., Currie D., Garte S. J. Activation of the K-ras oncogene in liver tumors of Hudson River tomcod. Carcinogenesis. 1989 Dec;10(12):2311–2315. doi: 10.1093/carcin/10.12.2311. [DOI] [PubMed] [Google Scholar]


