Abstract
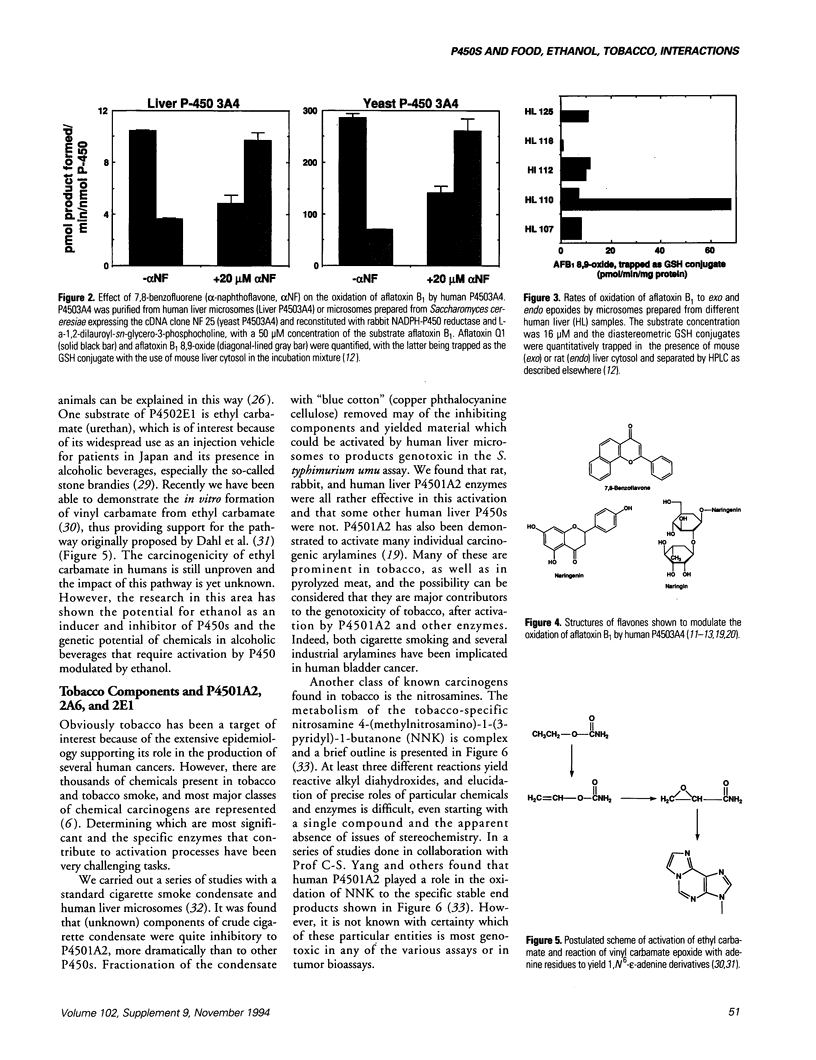
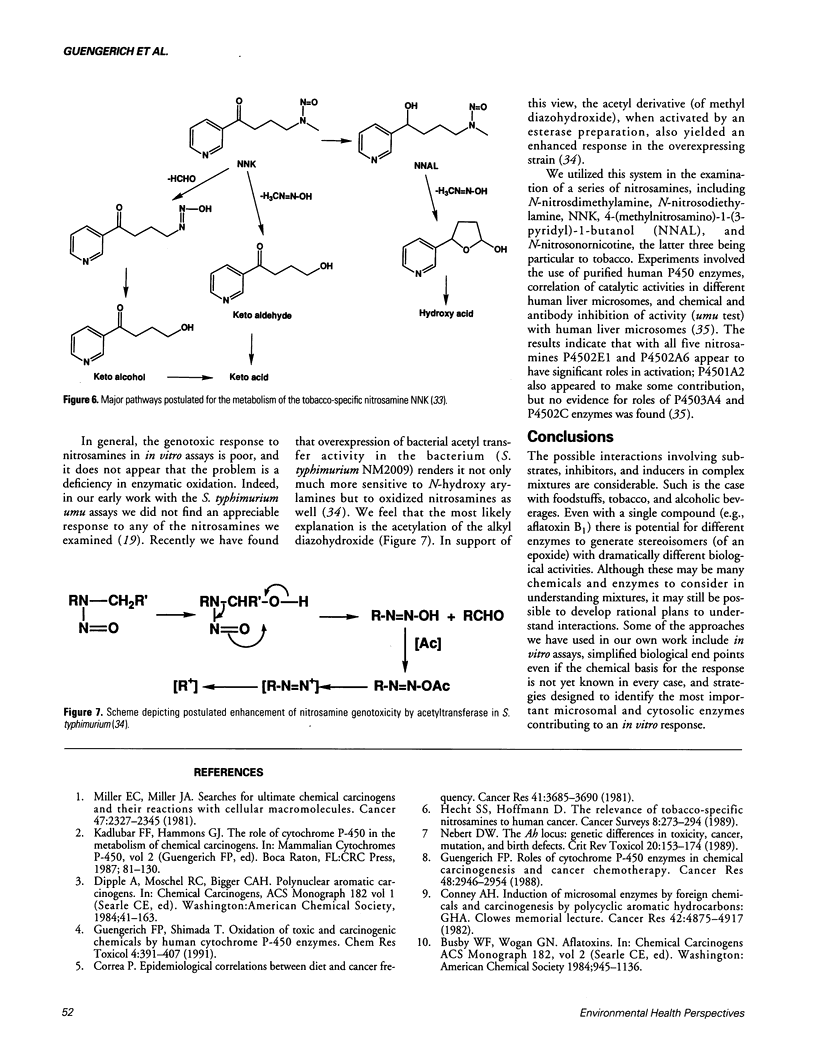
Human cytochrome P450 (P450) enzymes are involved in the oxidation of natural products found in foods, beverages, and tobacco products and their catalytic activities can also be modulated by components of the materials. The microsomal activation of aflatoxin B1 to the exo-8,9-epoxide is stimulated by flavone and 7,8-benzoflavone, and attenuated by the flavonoid naringenin, a major component of grapefruit. P4502E1 has been demonstrated to play a potentially major role in the activation of a number of very low-molecular weight cancer suspects, including ethyl carbamate (urethan), which is present in alcoholic beverages and particularly stone brandies. The enzyme (P4502E1) is also known to be inducible by ethanol. Tobacco contains a large number of potential carcinogens. In human liver microsomes a significant role for P4501A2 can be demonstrated in the activation of cigarette smoke condensate. Some of the genotoxicity may be due to arylamines. P4501A2 is also inhibited by components of crude cigarette smoke condensate. The tobacco-specific nitrosamines are activated by a number of P450 enzymes. Of those known to be present in human liver, P4501A2, 2A6, and 2E1 can activate these nitrosamines to genotoxic products.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bailey D. G., Spence J. D., Munoz C., Arnold J. M. Interaction of citrus juices with felodipine and nifedipine. Lancet. 1991 Feb 2;337(8736):268–269. doi: 10.1016/0140-6736(91)90872-m. [DOI] [PubMed] [Google Scholar]
- Buening M. K., Chang R. L., Huang M. T., Fortner J. G., Wood A. W., Conney A. H. Activation and inhibition of benzo(a)pyrene and aflatoxin B1 metabolism in human liver microsomes by naturally occurring flavonoids. Cancer Res. 1981 Jan;41(1):67–72. [PubMed] [Google Scholar]
- Conney A. H. Induction of microsomal enzymes by foreign chemicals and carcinogenesis by polycyclic aromatic hydrocarbons: G. H. A. Clowes Memorial Lecture. Cancer Res. 1982 Dec;42(12):4875–4917. [PubMed] [Google Scholar]
- Correa P. Epidemiological correlations between diet and cancer frequency. Cancer Res. 1981 Sep;41(9 Pt 2):3685–3690. [PubMed] [Google Scholar]
- Dahl G. A., Miller J. A., Miller E. C. Vinyl carbamate as a promutagen and a more carcinogenic analog of ethyl carbamate. Cancer Res. 1978 Nov;38(11 Pt 1):3793–3804. [PubMed] [Google Scholar]
- Guengerich F. P., Kim D. H. Enzymatic oxidation of ethyl carbamate to vinyl carbamate and its role as an intermediate in the formation of 1,N6-ethenoadenosine. Chem Res Toxicol. 1991 Jul-Aug;4(4):413–421. doi: 10.1021/tx00022a003. [DOI] [PubMed] [Google Scholar]
- Guengerich F. P., Kim D. H. In vitro inhibition of dihydropyridine oxidation and aflatoxin B1 activation in human liver microsomes by naringenin and other flavonoids. Carcinogenesis. 1990 Dec;11(12):2275–2279. doi: 10.1093/carcin/11.12.2275. [DOI] [PubMed] [Google Scholar]
- Guengerich F. P., Kim D. H., Iwasaki M. Role of human cytochrome P-450 IIE1 in the oxidation of many low molecular weight cancer suspects. Chem Res Toxicol. 1991 Mar-Apr;4(2):168–179. doi: 10.1021/tx00020a008. [DOI] [PubMed] [Google Scholar]
- Guengerich F. P. Roles of cytochrome P-450 enzymes in chemical carcinogenesis and cancer chemotherapy. Cancer Res. 1988 Jun 1;48(11):2946–2954. [PubMed] [Google Scholar]
- Guengerich F. P., Shimada T. Oxidation of toxic and carcinogenic chemicals by human cytochrome P-450 enzymes. Chem Res Toxicol. 1991 Jul-Aug;4(4):391–407. doi: 10.1021/tx00022a001. [DOI] [PubMed] [Google Scholar]
- Hecht S. S., Hoffmann D. The relevance of tobacco-specific nitrosamines to human cancer. Cancer Surv. 1989;8(2):273–294. [PubMed] [Google Scholar]
- Knodell R. G., Browne D. G., Gwozdz G. P., Brian W. R., Guengerich F. P. Differential inhibition of individual human liver cytochromes P-450 by cimetidine. Gastroenterology. 1991 Dec;101(6):1680–1691. doi: 10.1016/0016-5085(91)90408-d. [DOI] [PubMed] [Google Scholar]
- Koop D. R., Nordblom G. D., Coon M. J. Immunochemical evidence for a role of cytochrome P-450 in liver microsomal ethanol oxidation. Arch Biochem Biophys. 1984 Nov 15;235(1):228–238. doi: 10.1016/0003-9861(84)90272-8. [DOI] [PubMed] [Google Scholar]
- Koop D. R. Oxidative and reductive metabolism by cytochrome P450 2E1. FASEB J. 1992 Jan 6;6(2):724–730. doi: 10.1096/fasebj.6.2.1537462. [DOI] [PubMed] [Google Scholar]
- Lasker J. M., Huang M. T., Conney A. H. In vitro and in vivo activation of oxidative drug metabolism by flavonoids. J Pharmacol Exp Ther. 1984 Apr;229(1):162–170. [PubMed] [Google Scholar]
- Lieber C. S., DeCarli L. M. Hepatic microsomal ethanol-oxidizing system. In vitro characteristics and adaptive properties in vivo. J Biol Chem. 1970 May 25;245(10):2505–2512. [PubMed] [Google Scholar]
- Miller E. C., Miller J. A. Searches for ultimate chemical carcinogens and their reactions with cellular macromolecules. Cancer. 1981 May 15;47(10):2327–2345. doi: 10.1002/1097-0142(19810515)47:10<2327::aid-cncr2820471003>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Miller J. A. The need for epidemiological studies of the medical exposures of Japanese patients to the carcinogen ethyl carbamate (urethane) from 1950 to 1975. Jpn J Cancer Res. 1991 Dec;82(12):1323–1324. doi: 10.1111/j.1349-7006.1991.tb01799.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nebert D. W. The Ah locus: genetic differences in toxicity, cancer, mutation, and birth defects. Crit Rev Toxicol. 1989;20(3):153–174. doi: 10.3109/10408448909017908. [DOI] [PubMed] [Google Scholar]
- Orme-Johnson W. H., Ziegler D. M. Alcohol mixed function. Oxidase activity of mammalian liver microsomes. Biochem Biophys Res Commun. 1965 Oct 8;21(1):78–82. doi: 10.1016/0006-291x(65)90429-8. [DOI] [PubMed] [Google Scholar]
- Perrot N., Nalpas B., Yang C. S., Beaune P. H. Modulation of cytochrome P450 isozymes in human liver, by ethanol and drug intake. Eur J Clin Invest. 1989 Dec;19(6):549–555. doi: 10.1111/j.1365-2362.1989.tb00273.x. [DOI] [PubMed] [Google Scholar]
- Raney K. D., Coles B., Guengerich F. P., Harris T. M. The endo-8,9-epoxide of aflatoxin B1: a new metabolite. Chem Res Toxicol. 1992 May-Jun;5(3):333–335. doi: 10.1021/tx00027a002. [DOI] [PubMed] [Google Scholar]
- Raney K. D., Meyer D. J., Ketterer B., Harris T. M., Guengerich F. P. Glutathione conjugation of aflatoxin B1 exo- and endo-epoxides by rat and human glutathione S-transferases. Chem Res Toxicol. 1992 Jul-Aug;5(4):470–478. doi: 10.1021/tx00028a004. [DOI] [PubMed] [Google Scholar]
- Raney K. D., Shimada T., Kim D. H., Groopman J. D., Harris T. M., Guengerich F. P. Oxidation of aflatoxins and sterigmatocystin by human liver microsomes: significance of aflatoxin Q1 as a detoxication product of aflatoxin B1. Chem Res Toxicol. 1992 Mar-Apr;5(2):202–210. doi: 10.1021/tx00026a009. [DOI] [PubMed] [Google Scholar]
- Shimada T., Guengerich F. P. Activation of amino-alpha-carboline, 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine and a copper phthalocyanine cellulose extract of cigarette smoke condensate by cytochrome P-450 enzymes in rat and human liver microsomes. Cancer Res. 1991 Oct 1;51(19):5284–5291. [PubMed] [Google Scholar]
- Shimada T., Guengerich F. P. Evidence for cytochrome P-450NF, the nifedipine oxidase, being the principal enzyme involved in the bioactivation of aflatoxins in human liver. Proc Natl Acad Sci U S A. 1989 Jan;86(2):462–465. doi: 10.1073/pnas.86.2.462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimada T., Iwasaki M., Martin M. V., Guengerich F. P. Human liver microsomal cytochrome P-450 enzymes involved in the bioactivation of procarcinogens detected by umu gene response in Salmonella typhimurium TA 1535/pSK1002. Cancer Res. 1989 Jun 15;49(12):3218–3228. [PubMed] [Google Scholar]
- Smith T. J., Guo Z., Gonzalez F. J., Guengerich F. P., Stoner G. D., Yang C. S. Metabolism of 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone in human lung and liver microsomes and cytochromes P-450 expressed in hepatoma cells. Cancer Res. 1992 Apr 1;52(7):1757–1763. [PubMed] [Google Scholar]
- Yamazaki H., Inui Y., Yun C. H., Guengerich F. P., Shimada T. Cytochrome P450 2E1 and 2A6 enzymes as major catalysts for metabolic activation of N-nitrosodialkylamines and tobacco-related nitrosamines in human liver microsomes. Carcinogenesis. 1992 Oct;13(10):1789–1794. doi: 10.1093/carcin/13.10.1789. [DOI] [PubMed] [Google Scholar]
- Yamazaki H., Oda Y., Funae Y., Imaoka S., Inui Y., Guengerich F. P., Shimada T. Participation of rat liver cytochrome P450 2E1 in the activation of N-nitrosodimethylamine and N-nitrosodiethylamine to products genotoxic in an acetyltransferase-overexpressing Salmonella typhimurium strain (NM2009). Carcinogenesis. 1992 Jun;13(6):979–985. doi: 10.1093/carcin/13.6.979. [DOI] [PubMed] [Google Scholar]
- Yang C. S., Yoo J. S., Ishizaki H., Hong J. Y. Cytochrome P450IIE1: roles in nitrosamine metabolism and mechanisms of regulation. Drug Metab Rev. 1990;22(2-3):147–159. doi: 10.3109/03602539009041082. [DOI] [PubMed] [Google Scholar]


