Abstract
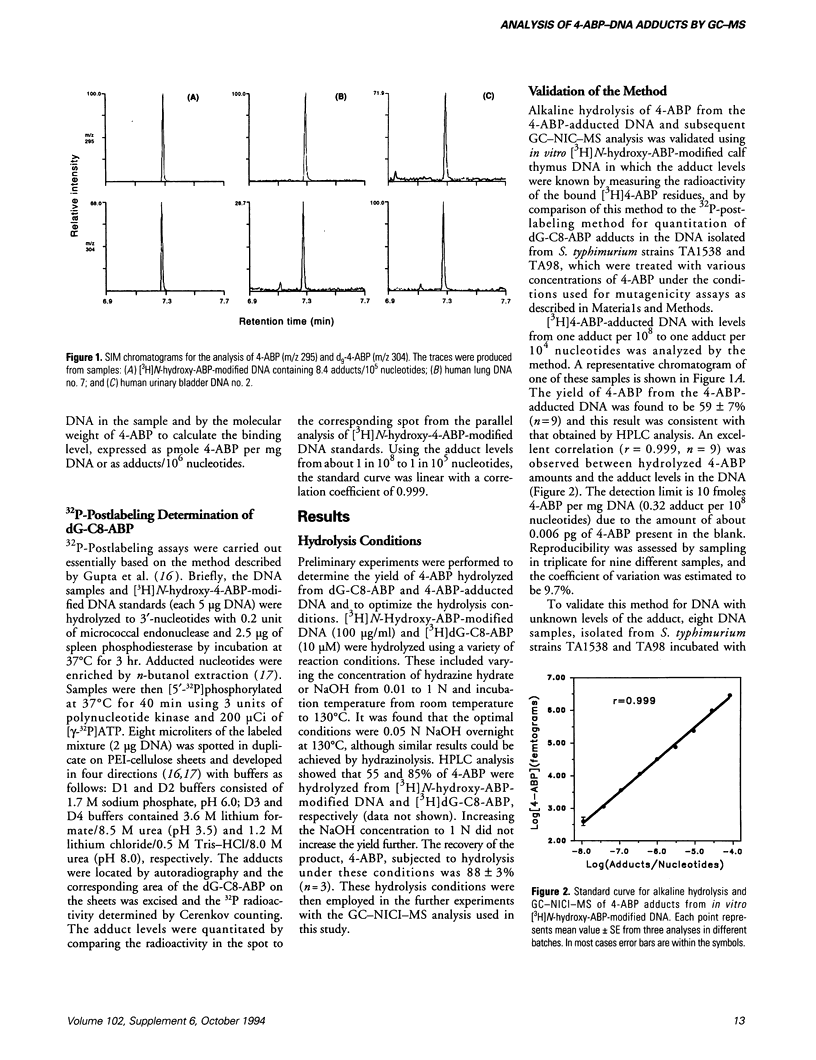
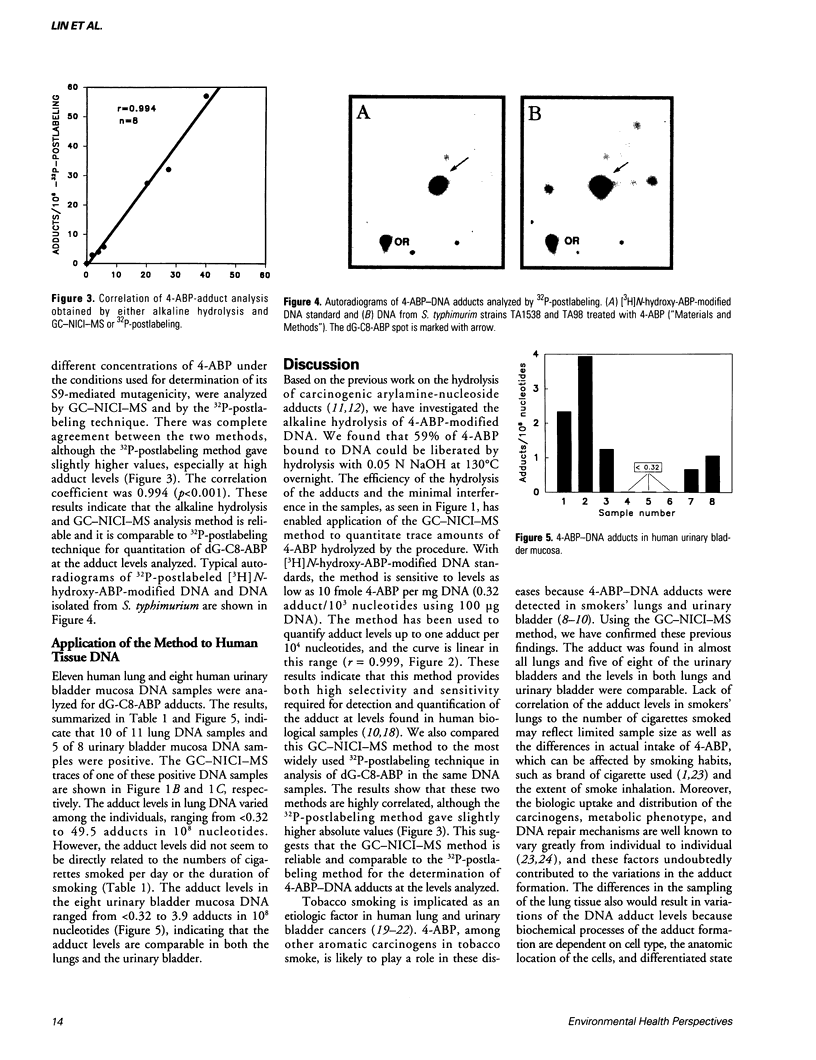
Analysis of carcinogen-DNA adducts has been regarded as a useful means of assessing human exposure to chemical carcinogens. We have established a method for quantitation of 4-aminobiphenyl (4-ABP)-DNA adducts by alkaline hydrolysis and gas chromatography with negative ion chemical ionization mass spectrometry (GC-NICI-MS). Aliquots of DNA (typically 100 micrograms/ml) were spiked with an internal standard, d9-4-ABP, and were hydrolyzed in 0.05 N NaOH at 130 degrees C overnight. The liberated 4-ABP was extracted with hexane and derivatized using pentafluoropropionic anhydride in trimethylamine for 30 min at room temperature prior to GC-NICI-MS. With in vitro [3H]N-hydroxy-4-ABP modified DNA standards, we observed 59 +/- 7% (n = 9) recovery of the 4-ABP and a linear correlation between hydrolyzed 4-ABP and the adduct levels ranging from about 1 in 10(8) to 1 in 10(4) nucleotides (r = 0.999, n = 9). The method was further validated by comparison of the results with that obtained by the 32P-postlabeling method. There was excellent agreement (r = 0.994, p < 0.001) between the two methods for quantitation of the adduct in eight samples of Salmonella typhimurium DNA treated with 4-ABP and rat liver S9, although the 32P-postlabeling method gave slightly higher values. The DNA adducts in 11 human lung and 8 urinary bladder mucosa specimens were then determined by our GC-NICI-MS method. The adduct levels were found to be < 0.32 to 49.5 adducts per 10(8) nucleotides in the lungs and < 0.32 to 3.94 adducts per 10(8) nucleotides in the bladder samples.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF



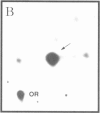
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Autrup H., Harris C. C., Stoner G. D., Selkirk J. K., Schafer P. W., Trump B. F. Metabolism of [3H]benzo[a]pyrene by cultured human bronchus and cultured human pulmonary alveolar macrophages. Lab Invest. 1978 Mar;38(3):217–224. [PubMed] [Google Scholar]
- Bakthavachalam J., Abdel-Baky S., Giese R. W. Release of 2-aminofluorene from N-(deoxyguanosin-8-yl)-2-aminofluorene by hydrazinolysis. J Chromatogr. 1991 Feb 1;538(2):447–451. doi: 10.1016/s0021-9673(01)88867-1. [DOI] [PubMed] [Google Scholar]
- Beland F. A., Beranek D. T., Dooley K. L., Heflich R. H., Kadlubar F. F. Arylamine-DNA adducts in vitro and in vivo: their role in bacterial mutagenesis and urinary bladder carcinogenesis. Environ Health Perspect. 1983 Mar;49:125–134. doi: 10.1289/ehp.8349125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belinsky S. A., White C. M., Devereux T. R., Swenberg J. A., Anderson M. W. Cell selective alkylation of DNA in rat lung following low dose exposure to the tobacco specific carcinogen 4-(N-methyl-N-nitrosamino)-1-(3-pyridyl)-1-butanone. Cancer Res. 1987 Feb 15;47(4):1143–1148. [PubMed] [Google Scholar]
- Bryant M. S., Skipper P. L., Tannenbaum S. R., Maclure M. Hemoglobin adducts of 4-aminobiphenyl in smokers and nonsmokers. Cancer Res. 1987 Jan 15;47(2):602–608. [PubMed] [Google Scholar]
- Bryant M. S., Vineis P., Skipper P. L., Tannenbaum S. R. Hemoglobin adducts of aromatic amines: associations with smoking status and type of tobacco. Proc Natl Acad Sci U S A. 1988 Dec;85(24):9788–9791. doi: 10.1073/pnas.85.24.9788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clavel J., Cordier S., Boccon-Gibod L., Hemon D. Tobacco and bladder cancer in males: increased risk for inhalers and smokers of black tobacco. Int J Cancer. 1989 Oct 15;44(4):605–610. doi: 10.1002/ijc.2910440408. [DOI] [PubMed] [Google Scholar]
- Coghlin J., Gann P. H., Hammond S. K., Skipper P. L., Taghizadeh K., Paul M., Tannenbaum S. R. 4-Aminobiphenyl hemoglobin adducts in fetuses exposed to the tobacco smoke carcinogen in utero. J Natl Cancer Inst. 1991 Feb 20;83(4):274–280. doi: 10.1093/jnci/83.4.274. [DOI] [PubMed] [Google Scholar]
- Del Santo P., Moneti G., Salvadori M., Saltutti C., Delle Rose A., Dolara P. Levels of the adducts of 4-aminobiphenyl to hemoglobin in control subjects and bladder carcinoma patients. Cancer Lett. 1991 Dec 1;60(3):245–251. doi: 10.1016/0304-3835(91)90120-7. [DOI] [PubMed] [Google Scholar]
- Doll R., Peto R. Cigarette smoking and bronchial carcinoma: dose and time relationships among regular smokers and lifelong non-smokers. J Epidemiol Community Health. 1978 Dec;32(4):303–313. doi: 10.1136/jech.32.4.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta R. C. Enhanced sensitivity of 32P-postlabeling analysis of aromatic carcinogen:DNA adducts. Cancer Res. 1985 Nov;45(11 Pt 2):5656–5662. [PubMed] [Google Scholar]
- Gupta R. C. Nonrandom binding of the carcinogen N-hydroxy-2-acetylaminofluorene to repetitive sequences of rat liver DNA in vivo. Proc Natl Acad Sci U S A. 1984 Nov;81(22):6943–6947. doi: 10.1073/pnas.81.22.6943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta R. C., Reddy M. V., Randerath K. 32P-postlabeling analysis of non-radioactive aromatic carcinogen--DNA adducts. Carcinogenesis. 1982;3(9):1081–1092. doi: 10.1093/carcin/3.9.1081. [DOI] [PubMed] [Google Scholar]
- Harris C. C. Interindividual variation among humans in carcinogen metabolism, DNA adduct formation and DNA repair. Carcinogenesis. 1989 Sep;10(9):1563–1566. doi: 10.1093/carcin/10.9.1563. [DOI] [PubMed] [Google Scholar]
- Malaveille C., Vineis P., Estéve J., Ohshima H., Brun G., Hautefeuille A., Gallet P., Ronco G., Terracini B., Bartsch H. Levels of mutagens in the urine of smokers of black and blond tobacco correlate with their risk of bladder cancer. Carcinogenesis. 1989 Mar;10(3):577–586. doi: 10.1093/carcin/10.3.577. [DOI] [PubMed] [Google Scholar]
- Peluso M., Castegnaro M., Malaveille C., Friesen M., Garren L., Hautefeuille A., Vineis P., Kadlubar F., Bartsch H. 32P Postlabelling analysis of urinary mutagens from smokers of black tobacco implicates 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine (PhIP) as a major DNA-damaging agent. Carcinogenesis. 1991 Apr;12(4):713–717. doi: 10.1093/carcin/12.4.713. [DOI] [PubMed] [Google Scholar]
- Talaska G., Schamer M., Skipper P., Tannenbaum S., Caporaso N., Unruh L., Kadlubar F. F., Bartsch H., Malaveille C., Vineis P. Detection of carcinogen-DNA adducts in exfoliated urothelial cells of cigarette smokers: association with smoking, hemoglobin adducts, and urinary mutagenicity. Cancer Epidemiol Biomarkers Prev. 1991 Nov-Dec;1(1):61–66. [PubMed] [Google Scholar]
- Talaska G., al-Juburi A. Z., Kadlubar F. F. Smoking related carcinogen-DNA adducts in biopsy samples of human urinary bladder: identification of N-(deoxyguanosin-8-yl)-4-aminobiphenyl as a major adduct. Proc Natl Acad Sci U S A. 1991 Jun 15;88(12):5350–5354. doi: 10.1073/pnas.88.12.5350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vineis P., Caporaso N., Tannenbaum S. R., Skipper P. L., Glogowski J., Bartsch H., Coda M., Talaska G., Kadlubar F. Acetylation phenotype, carcinogen-hemoglobin adducts, and cigarette smoking. Cancer Res. 1990 May 15;50(10):3002–3004. [PubMed] [Google Scholar]
- Vineis P., Estève J., Terracini B. Bladder cancer and smoking in males: types of cigarettes, age at start, effect of stopping and interaction with occupation. Int J Cancer. 1984 Aug 15;34(2):165–170. doi: 10.1002/ijc.2910340205. [DOI] [PubMed] [Google Scholar]
- Wilson V. L., Weston A., Manchester D. K., Trivers G. E., Roberts D. W., Kadlubar F. F., Wild C. P., Montesano R., Willey J. C., Mann D. L. Alkyl and aryl carcinogen adducts detected in human peripheral lung. Carcinogenesis. 1989 Nov;10(11):2149–2153. doi: 10.1093/carcin/10.11.2149. [DOI] [PubMed] [Google Scholar]