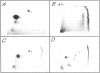
Abstract
The mutagenicity, metabolism, DNA adduction and induction of unscheduled DNA synthesis (UDS) of 1-nitropyrene and 1,8-dinitropyrene were investigated in the human hepatoma cell line HepG2. Previous results had demonstrated that 1-nitropyrene was both mutagenic at the hgprt locus and induced UDS in these cells. In the present study, we find that the dinitropyrenes, although highly mutagenic in Salmonella typhimurium, are not mutagenic and do not induce UDS in the HepG2. Although the rate of 1,8-dinitropyrene nitroreduction was less than that of 1-nitropyrene nitroreduction, this did not explain the lack of mutagenicity and UDS induction by the dinitropyrenes. Therefore, it is proposed that the arylhydroxylamine O-esterificase is not expressed in these cells. Since cytochrome P450-mediated C-oxidation is the predominant metabolic pathway in vivo, we sought to determine if an increase in the ratio of cytochrome P450-mediated C-oxidation over nitroreduction would result in increased or decreased DNA adducts in the HepG2. The administration of 2.5 microM 3-methylcholanthrene to the HepG2 increased the ratio of C-oxidation/nitroreduction from 2.8 +/- 1.9 to 50.4 +/- 46.1. This was accompanied by a decrease in the C8-guanyl adduct of 1-nitropyrene (via nitroreduction) from 18.7 +/- 7.0 to 4.8 +/- 1.7 fmoles/micrograms DNA, without any further increase in other 1-nitropyrene DNA adducts. These results suggest that the cytochrome P450-mediated metabolism of 1-nitropyrene to epoxides, phenols, and dihydrodiols is not an activation pathway in the HepG2 cells, and may explain the weak carcinogenicity of 1-nitropyrene in vivo, where cytochrome P450-mediated C-oxidation predominates.
Full text
PDF
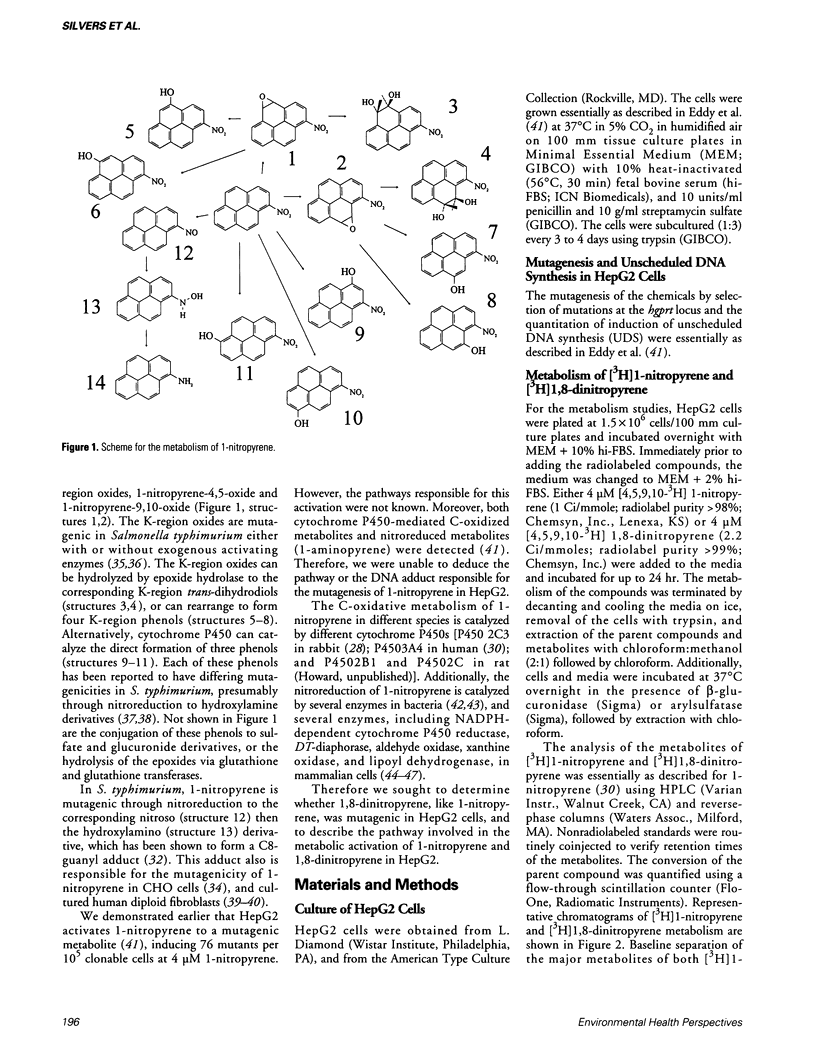
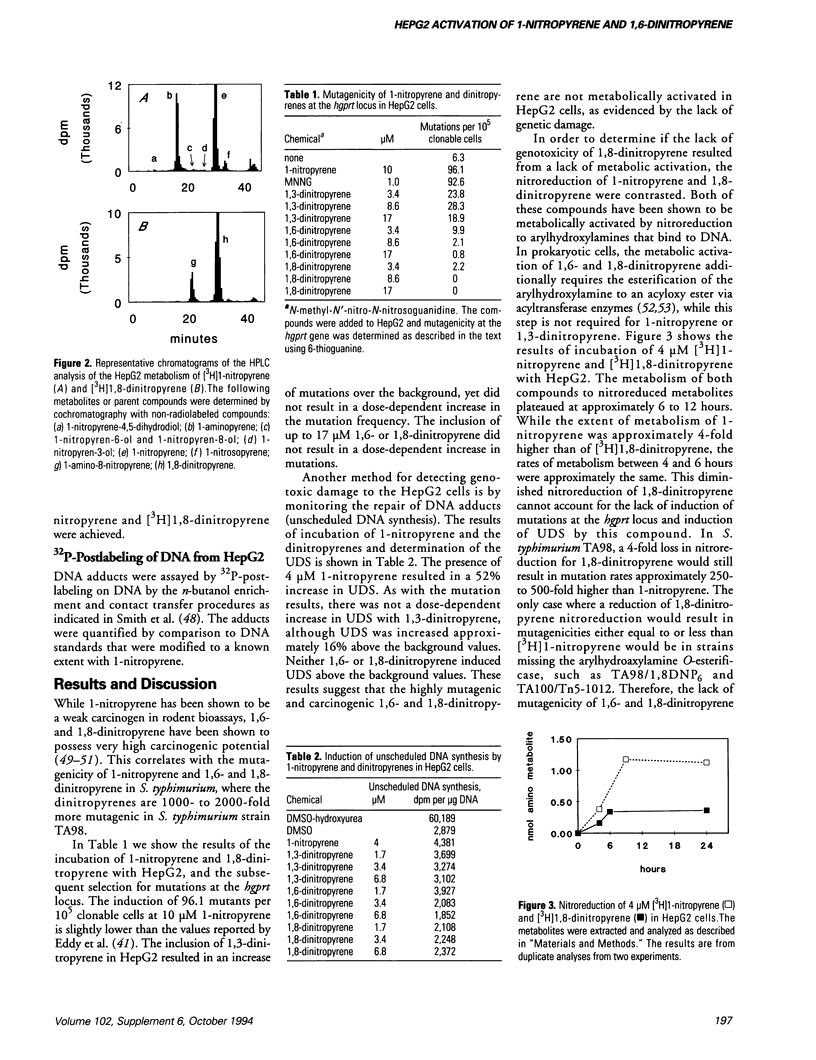
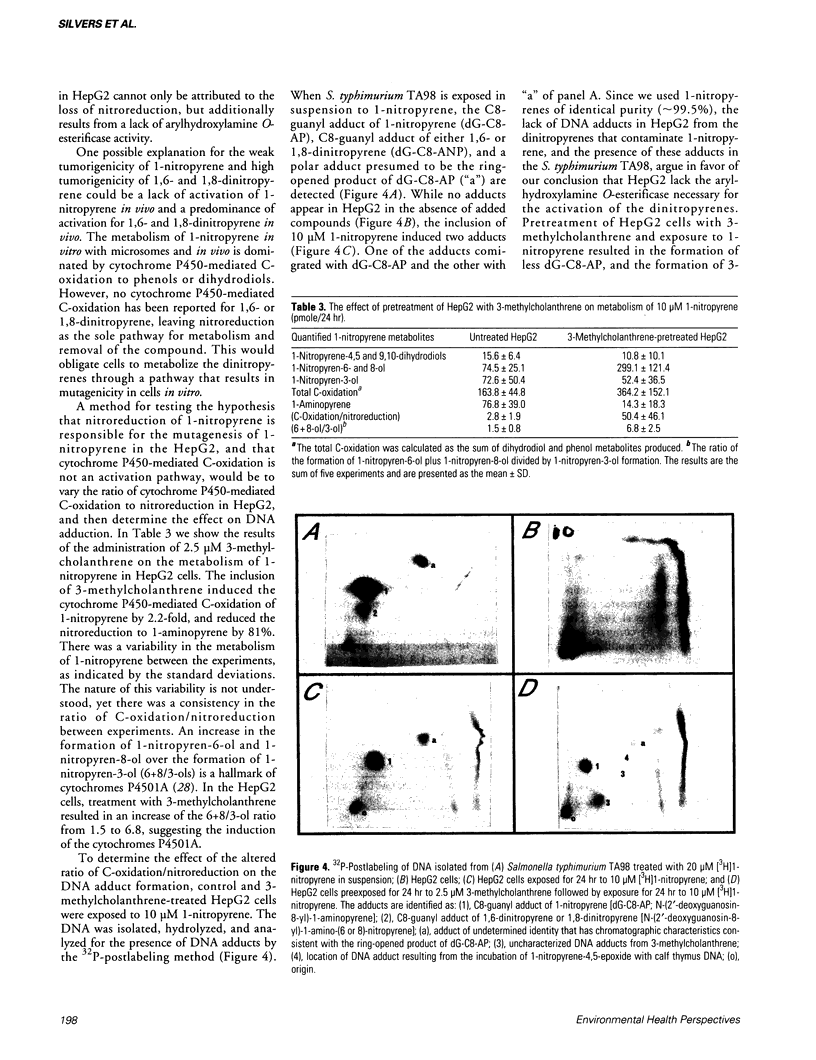


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aden D. P., Fogel A., Plotkin S., Damjanov I., Knowles B. B. Controlled synthesis of HBsAg in a differentiated human liver carcinoma-derived cell line. Nature. 1979 Dec 6;282(5739):615–616. doi: 10.1038/282615a0. [DOI] [PubMed] [Google Scholar]
- Babich H., Sardana M. K., Borenfreund E. Acute cytotoxicities of polynuclear aromatic hydrocarbons determined in vitro with the human liver tumor cell line, HepG2. Cell Biol Toxicol. 1988 Sep;4(3):295–309. doi: 10.1007/BF00058738. [DOI] [PubMed] [Google Scholar]
- Ball L. M., Kohan M. J., Inmon J. P., Claxton L. D., Lewtas J. Metabolism of 1-nitro[14C]pyrene in vivo in the rat and mutagenicity of urinary metabolites. Carcinogenesis. 1984 Dec;5(12):1557–1564. doi: 10.1093/carcin/5.12.1557. [DOI] [PubMed] [Google Scholar]
- Bauer S. L., Howard P. C. Kinetics and cofactor requirements for the nitroreductive metabolism of 1-nitropyrene and 3-nitrofluoranthene by rabbit liver aldehyde oxidase. Carcinogenesis. 1991 Sep;12(9):1545–1549. doi: 10.1093/carcin/12.9.1545. [DOI] [PubMed] [Google Scholar]
- Bauer S. L., Howard P. C. The kinetics of 1-nitropyrene and 3-nitrofluoranthene metabolism using bovine liver xanthine oxidase. Cancer Lett. 1990 Oct 8;54(1-2):37–42. doi: 10.1016/0304-3835(90)90088-f. [DOI] [PubMed] [Google Scholar]
- Beland F. A., Ribovich M., Howard P. C., Heflich R. H., Kurian P., Milo G. E. Cytotoxicity, cellular transformation and DNA adducts in normal human diploid fibroblasts exposed to 1-nitrosopyrene, a reduced derivative of the environmental contaminant, 1-nitropyrene. Carcinogenesis. 1986 Aug;7(8):1279–1283. doi: 10.1093/carcin/7.8.1279. [DOI] [PubMed] [Google Scholar]
- Bhatt T. S., Coombs M., DiGiovanni J., Diamond L. Mutagenesis in Chinese hamster cells by cyclopenta(a)phenanthrenes activated by a human hepatoma cell line. Cancer Res. 1983 Mar;43(3):984–996. [PubMed] [Google Scholar]
- Busch S. J., Martin G. A., Barnhart R. L., Jackson R. L. Heparin induces the expression of hepatic triglyceride lipase in a human hepatoma (HepG2) cell line. J Biol Chem. 1989 Jun 5;264(16):9527–9532. [PubMed] [Google Scholar]
- Chang G., Jacobson-Kram D., Williams J. R. Use of an established human hepatoma cell line with endogenous bioactivation for gene mutation studies. Cell Biol Toxicol. 1988 Sep;4(3):267–279. doi: 10.1007/BF00058736. [DOI] [PubMed] [Google Scholar]
- Cohen L. H., Griffioen M., van Roermund C. W., Wanders R. J. Subcellular localization of squalene synthase in human hepatoma cell line Hep G2. Biochim Biophys Acta. 1992 Jun 5;1126(1):114–118. doi: 10.1016/0005-2760(92)90224-j. [DOI] [PubMed] [Google Scholar]
- Consolo M. C., Anders M., Howard P. C. Mutagenicity of the phenolic microsomal metabolites of 3-nitrofluoranthene and 1-nitropyrene in strains of Salmonella typhimurium. Mutat Res. 1989 Feb;210(2):263–269. doi: 10.1016/0027-5107(89)90087-0. [DOI] [PubMed] [Google Scholar]
- Dearfield K. L., Jacobson-Kram D., Brown N. A., Williams J. R. Evaluation of a human hepatoma cell line as a target cell in genetic toxicology. Mutat Res. 1983 Mar;108(1-3):437–449. doi: 10.1016/0027-5107(83)90138-0. [DOI] [PubMed] [Google Scholar]
- DiGiovanni J., Singer J. M., Diamond L. Comparison of the metabolic activation of 7, 12-dimethylbenz(a)anthracene by a human hepatoma cell line (HepG2) and low passage hamster embryo cells. Cancer Res. 1984 Jul;44(7):2878–2884. [PubMed] [Google Scholar]
- Diamond L., Cherian K., Harvey R. G., DiGiovanni J. Mutagenic activity of methyl- and fluoro-substituted derivatives of polycyclic aromatic hydrocarbons in a human hepatoma (HepG2) cell-mediated assay. Mutat Res. 1984 Apr;136(1):65–72. doi: 10.1016/0165-1218(84)90135-6. [DOI] [PubMed] [Google Scholar]
- Diamond L., Kruszewski F., Aden D. P., Knowles B. B., Baird W. M. Metabolic activation of benzo[a]pyrene by a human hepatoma cell line. Carcinogenesis. 1980;1(10):871–875. doi: 10.1093/carcin/1.10.871. [DOI] [PubMed] [Google Scholar]
- Djurić Z., Fifer E. K., Howard P. C., Beland F. A. Oxidative microsomal metabolism of 1-nitropyrene and DNA-binding of oxidized metabolites following nitroreduction. Carcinogenesis. 1986 Jul;7(7):1073–1079. doi: 10.1093/carcin/7.7.1073. [DOI] [PubMed] [Google Scholar]
- Djurić Z., Potter D. W., Heflich R. H., Beland F. A. Aerobic and anaerobic reduction of nitrated pyrenes in vitro. Chem Biol Interact. 1986 Oct 1;59(3):309–324. doi: 10.1016/s0009-2797(86)80076-x. [DOI] [PubMed] [Google Scholar]
- Eddy E. P., Howard P. C., McCoy G. D., Rosenkranz H. S. Mutagenicity, unscheduled DNA synthesis, and metabolism of 1-nitropyrene in the human hepatoma cell line HepG2. Cancer Res. 1987 Jun 15;47(12):3163–3168. [PubMed] [Google Scholar]
- Everson G. T., Polokoff M. A. HepG2. A human hepatoblastoma cell line exhibiting defects in bile acid synthesis and conjugation. J Biol Chem. 1986 Feb 15;261(5):2197–2201. [PubMed] [Google Scholar]
- Faust R. A., Cheung M. C., Albers J. J. Secretion of cholesteryl ester transfer protein-lipoprotein complexes by human HepG2 hepatocytes. Atherosclerosis. 1989 May;77(1):77–82. doi: 10.1016/0021-9150(89)90012-9. [DOI] [PubMed] [Google Scholar]
- Fuki I. V., Preobrazhensky S. N., Misharin AYu, Bushmakina N. G., Menschikov G. B., Repin V. S., Karpov R. S. Effect of cell cholesterol content on apolipoprotein B secretion and LDL receptor activity in the human hepatoma cell line, HepG2. Biochim Biophys Acta. 1989 Feb 6;1001(2):235–238. doi: 10.1016/0005-2760(89)90153-7. [DOI] [PubMed] [Google Scholar]
- Heflich R. H., Fullerton N. F., Beland F. A. An examination of the weak mutagenic response of 1-nitropyrene in Chinese hamster ovary cells. Mutat Res. 1986 Jun;161(1):99–108. doi: 10.1016/0027-5107(86)90104-1. [DOI] [PubMed] [Google Scholar]
- Heflich R. H., Howard P. C., Beland F. A. 1-Nitrosopyrene: an intermediate in the metabolic activation of 1-nitropyrene to a mutagen in Salmonella typhimurium TA1538. Mutat Res. 1985 Mar;149(1):25–32. doi: 10.1016/0027-5107(85)90005-3. [DOI] [PubMed] [Google Scholar]
- Howard P. C., Aoyama T., Bauer S. L., Gelboin H. V., Gonzalez F. J. The metabolism of 1-nitropyrene by human cytochromes P450. Carcinogenesis. 1990 Sep;11(9):1539–1542. doi: 10.1093/carcin/11.9.1539. [DOI] [PubMed] [Google Scholar]
- Howard P. C., Beland F. A. Xanthine oxidase catalyzed binding of 1-nitropyrene to DNA. Biochem Biophys Res Commun. 1982 Jan 29;104(2):727–732. doi: 10.1016/0006-291x(82)90697-0. [DOI] [PubMed] [Google Scholar]
- Howard P. C., Flammang T. J., Beland F. A. Comparison of the in vitro and in vivo hepatic metabolism of the carcinogen 1-nitropyrene. Carcinogenesis. 1985 Feb;6(2):243–249. doi: 10.1093/carcin/6.2.243. [DOI] [PubMed] [Google Scholar]
- Howard P. C., Gerrard J. A., Milo G. E., Fu P. P., Beland F. A., Kadlubar F. F. Transformation of normal human skin fibroblasts by 1-nitropyrene and 6-nitrobenzo[a]pyrene. Carcinogenesis. 1983;4(3):353–355. doi: 10.1093/carcin/4.3.353. [DOI] [PubMed] [Google Scholar]
- Howard P. C., Heflich R. H., Evans F. E., Beland F. A. Formation of DNA adducts in vitro and in Salmonella typhimurium upon metabolic reduction of the environmental mutagen 1-nitropyrene. Cancer Res. 1983 May;43(5):2052–2058. [PubMed] [Google Scholar]
- Howard P. C., Reed K. A., Koop D. R. Oxidative metabolism of 1-nitropyrene by rabbit liver microsomes and purified microsomal cytochrome P-450 isozymes. Cancer Res. 1988 Aug 1;48(15):4261–4265. [PubMed] [Google Scholar]
- Huber B. E., Dearfield K. L., Williams J. R., Heilman C. A., Thorgeirsson S. S. Tumorigenicity and transcriptional modulation of c-myc and N-ras oncogenes in a human hepatoma cell line. Cancer Res. 1985 Sep;45(9):4322–4329. [PubMed] [Google Scholar]
- Imaida K., Hirose M., Tay L., Lee M. S., Wang C. Y., King C. M. Comparative carcinogenicities of 1-, 2-, and 4-nitropyrene and structurally related compounds in the female CD rat. Cancer Res. 1991 Jun 1;51(11):2902–2907. [PubMed] [Google Scholar]
- Iwasa F., Sassa S., Kappas A. delta-Aminolaevulinate synthase in human HepG2 hepatoma cells. Repression by haemin and induction by chemicals. Biochem J. 1989 Sep 15;262(3):807–813. doi: 10.1042/bj2620807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kataoka K., Kinouchi T., Ohnishi Y. Species differences in metabolic activation and inactivation of 1-nitropyrene in the liver. Cancer Res. 1991 Aug 1;51(15):3919–3924. [PubMed] [Google Scholar]
- Knowles B. B., Howe C. C., Aden D. P. Human hepatocellular carcinoma cell lines secrete the major plasma proteins and hepatitis B surface antigen. Science. 1980 Jul 25;209(4455):497–499. doi: 10.1126/science.6248960. [DOI] [PubMed] [Google Scholar]
- Lescoat G., Desvergne B., Loreal O., Pasdeloup N., Deugnier Y., Bourel M., Brissot P. Modulation of albumin secretion by ornithine alpha-ketoglutarate in adult rat hepatocyte cultures and a human hepatoma cell line (HepG2). Ann Nutr Metab. 1989;33(5):252–260. doi: 10.1159/000177542. [DOI] [PubMed] [Google Scholar]
- McCoy E. C., Anders M., Rosenkranz H. S. The basis of the insensitivity of Salmonella typhimurium strain TA98/1,8-DNP6 to the mutagenic action of nitroarenes. Mutat Res. 1983 Jul;121(1):17–23. doi: 10.1016/0165-7992(83)90081-7. [DOI] [PubMed] [Google Scholar]
- McCoy E. C., Rosenkranz H. S., Howard P. C. Salmonella typhimurium TA100Tn5-1012, a strain deficient in arylhydroxylamine O-esterificase, exhibits a reduced nitroreductase activity. Mutat Res. 1990 Feb;243(2):141–144. doi: 10.1016/0165-7992(90)90036-j. [DOI] [PubMed] [Google Scholar]
- Ohgaki H., Hasegawa H., Kato T., Negishi C., Sato S., Sugimura T. Absence of carcinogenicity of 1-nitropyrene, correction of previous results, and new demonstration of carcinogenicity of 1,6-dinitropyrene in rats. Cancer Lett. 1985 Jan;25(3):239–245. doi: 10.1016/s0304-3835(15)30002-1. [DOI] [PubMed] [Google Scholar]
- Orr J. C., Bryant D. W., McCalla D. R., Quilliam M. A. Dinitropyrene-resistant Salmonella typhimurium are deficient in an acetyl-CoA acetyltransferase. Chem Biol Interact. 1985 Aug-Sep;54(3):281–288. doi: 10.1016/s0009-2797(85)80169-1. [DOI] [PubMed] [Google Scholar]
- Rosenkranz H. S., McCoy E. C., Mermelstein R., Speck W. T. A cautionary note the use of nitroreductase-deficient strains of Salmonella typhimurium for the detection of nitroarenes as mutagens in complex mixtures including diesel exhausts. Mutat Res. 1981 Mar;91(2):103–105. doi: 10.1016/0165-7992(81)90080-4. [DOI] [PubMed] [Google Scholar]
- Saito H., Goodnough L. T., Knowles B. B., Aden D. P. Synthesis and secretion of alpha 2-plasmin inhibitor by established human liver cell lines. Proc Natl Acad Sci U S A. 1982 Sep;79(18):5684–5687. doi: 10.1073/pnas.79.18.5684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito K., Kamataki T., Kato R. Participation of cytochrome P-450 in reductive metabolism of 1-nitropyrene by rat liver microsomes. Cancer Res. 1984 Aug;44(8):3169–3173. [PubMed] [Google Scholar]
- Silvers K. J., Chazinski T., McManus M. E., Bauer S. L., Gonzalez F. J., Gelboin H. V., Maurel P., Howard P. C. Cytochrome P-450 3A4 (nifedipine oxidase) is responsible for the C-oxidative metabolism of 1-nitropyrene in human liver microsomal samples. Cancer Res. 1992 Nov 15;52(22):6237–6243. [PubMed] [Google Scholar]
- Smith B. A., Korfmacher W. A., Beland F. A. DNA adduct formation in target tissues of Sprague-Dawley rats, CD-1 mice and A/J mice following tumorigenic doses of 1-nitropyrene. Carcinogenesis. 1990 Oct;11(10):1705–1710. doi: 10.1093/carcin/11.10.1705. [DOI] [PubMed] [Google Scholar]
- Strous G. J., Van Kerkhof P. Release of soluble resident as well as secretory proteins from HepG2 cells by partial permeabilization of rough-endoplasmic-reticulum membranes. Biochem J. 1989 Jan 1;257(1):159–163. doi: 10.1042/bj2570159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokiwa H., Ohnishi Y. Mutagenicity and carcinogenicity of nitroarenes and their sources in the environment. Crit Rev Toxicol. 1986;17(1):23–60. doi: 10.3109/10408448609037070. [DOI] [PubMed] [Google Scholar]
- Winkler K. E., Harrison E. H., Marsh J. B., Glick J. M., Ross A. C. Characterization of a bile salt-dependent cholesteryl ester hydrolase activity secreted from HepG2 cells. Biochim Biophys Acta. 1992 Jun 22;1126(2):151–158. doi: 10.1016/0005-2760(92)90285-4. [DOI] [PubMed] [Google Scholar]
- Wong S. H., Fisher E. A., Marsh J. B. Effects of eicosapentaenoic and docosahexaenoic acids on apoprotein B mRNA and secretion of very low density lipoprotein in HepG2 cells. Arteriosclerosis. 1989 Nov-Dec;9(6):836–841. doi: 10.1161/01.atv.9.6.836. [DOI] [PubMed] [Google Scholar]
- Zannis V. I., Breslow J. L., SanGiacomo T. R., Aden D. P., Knowles B. B. Characterization of the major apolipoproteins secreted by two human hepatoma cell lines. Biochemistry. 1981 Dec 8;20(25):7089–7096. doi: 10.1021/bi00528a006. [DOI] [PubMed] [Google Scholar]
- Zhuo Z., Casciano D. A., Heflich R. H. Use of the human liver cell line Hep G2 in a modified Salmonella reversion assay. Cancer Lett. 1986 Sep;32(3):327–334. doi: 10.1016/0304-3835(86)90186-2. [DOI] [PubMed] [Google Scholar]