Case
A 60-year-old woman with diabetes mellitus, hypertension and known kidney disease presents to her family physician for a routine assessment. She has mild fatigue but is otherwise asymptomatic. Her weight is 55 kg, and her blood pressure is 155/90 mm Hg with no postural changes. She has no peripheral or periorbital edema. Findings on precordial examination are unremarkable, but there are diffusely reduced peripheral pulses and a right femoral bruit. Funduscopy reveals arteriovenous nicking, the appearance of silver wiring and occasional cotton-wool exudates. The patient's current medications include hydrochlorothiazide (25 mg/d) and insulin (subcutaneously, twice daily). Her most recent serum creatinine values, measured 12 and 18 months ago, were 140 μmol/L and 132 μmol/L respectively.
How should the patient's degree of kidney dysfunction be assessed? What biochemical abnormalities are characteristic of kidney disease? How can the patient's medical management be optimized? When should she be referred to a nephrologist?
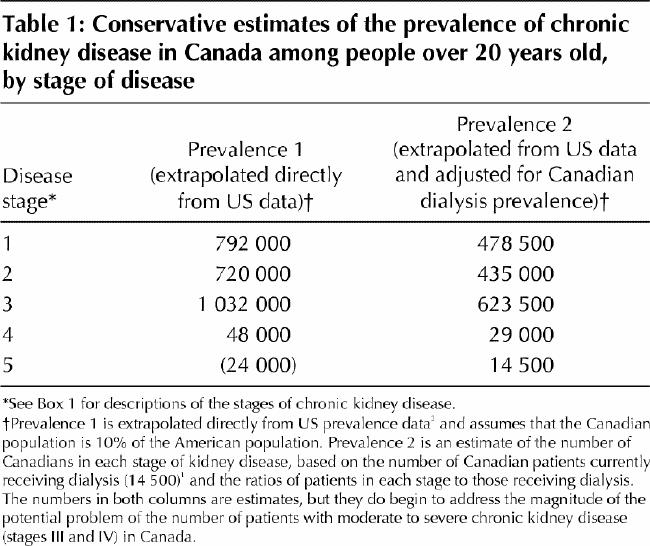
The incidence of end-stage renal disease (ESRD) is increasing at an annual rate of about 7%, with a corresponding growth in the prevalence of Canadians requiring dialysis from 223 per million population in 1981 to 810 per million in 2000.1 The number of patients who have chronic kidney disease but who do not require renal replacement therapy is unknown. It is estimated that at least 600 000 to 1 million people have stage III (moderate) or worse kidney disease,2 as demonstrated by a glomerular filtration rate below 60 mL/min (1.00 mL/s) per 1.73 m2. Table 1 shows the number of patients by stage of chronic kidney disease, estimated from both US and Canadian prevalent dialysis populations. The stages of chronic kidney disease, as classified by the US National Kidney Foundation,2 are defined in Box 1.
Table 1
Box 1.
Kidney disease at all stages is associated with a substantial burden of illness. There is accumulating data suggesting that even mild levels of kidney dysfunction are associated with worse outcomes.3 In the past 2 decades, improvements in the medical management of kidney disease have delayed the rate of progression to kidney failure.4 In addition, referral to a nephrologist has been found to be associated with improved clinical outcomes, as demonstrated by minimized biochemical abnormalities, decreased use of temporary devices for vascular access and shortened hospital stays at the time of initiation of renal replacement therapy.5,6 The poor outcomes of many patients receiving dialysis (about 50% of prevalent dialysis patients die within 5 years7) are often due to the substantial burden of illness that accrues in these patients as their condition progresses to ESRD. Optimal care before dialysis may thus ultimately have an impact on the survival of chronic dialysis patients. In fact, Gorriz and coworkers8 recently showed that longer nephrological care before dialysis was associated with improved long-term survival of dialysis patients.
Primary care physicians are positioned to recognize the early stages of kidney disease and to subsequently include nephrologists and associated health care professionals in the care of these patients. Recently, guidelines have been implemented for the management and referral of patients with elevated serum creatinine levels.9 Furthermore, a new classification system that helps to simplify and identify patients with chronic kidney disease has been published.2 In this article, we provide an approach to the identification and management of factors associated with the progression of kidney disease and of specific complications of chronic renal insufficiency.
Diagnosis
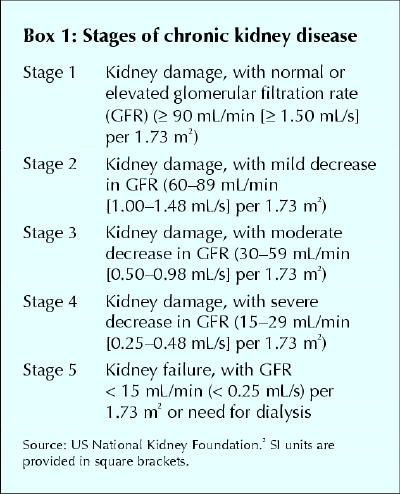
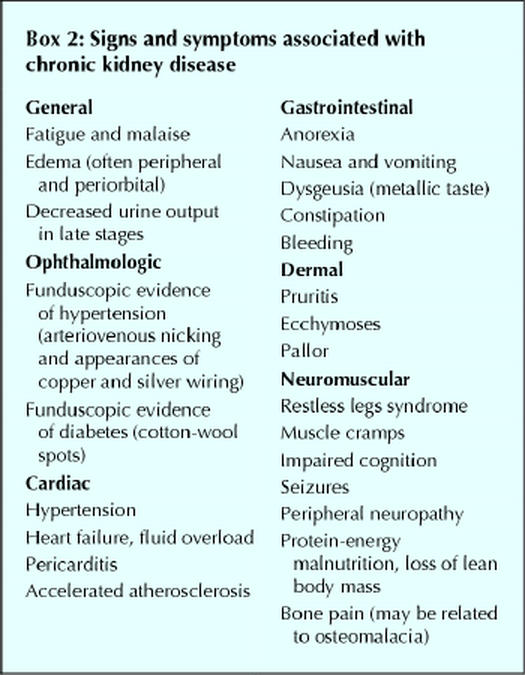
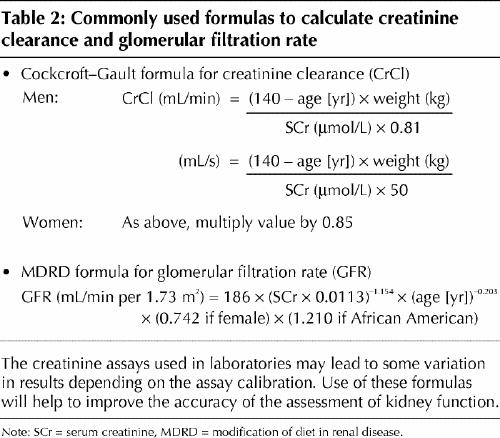
The diagnosis of chronic kidney disease is often delayed because the signs, symptoms and metabolic abnormalities associated with kidney impairment are insidious and nonspecific (Boxes 2 and 3) and because the serum creatinine level, by which renal function is often assessed, is an insensitive marker of kidney impairment. The dependence of the serum creatinine level on muscle mass makes women and older patients particularly susceptible to missed diagnoses. We recommend the use of a more sensitive index of renal function, the creatinine clearance. This parameter can be determined by measurement of creatinine in a 24-hour urine collection (with a concurrent serum creatinine measurement) or alternatively, and more easily, by calculation using the Cockcroft–Gault formula10 (Table 2). A normal glomerular filtration rate is 120 (standard deviation [SD] 20) mL/min (2.00 [SD 0.33] mL/s) per 1.73 m2. Abnormal glomerular filtration rates are used to classify the stages of chronic kidney disease (Box 1).
Box 2.
Box 3.
Table 2
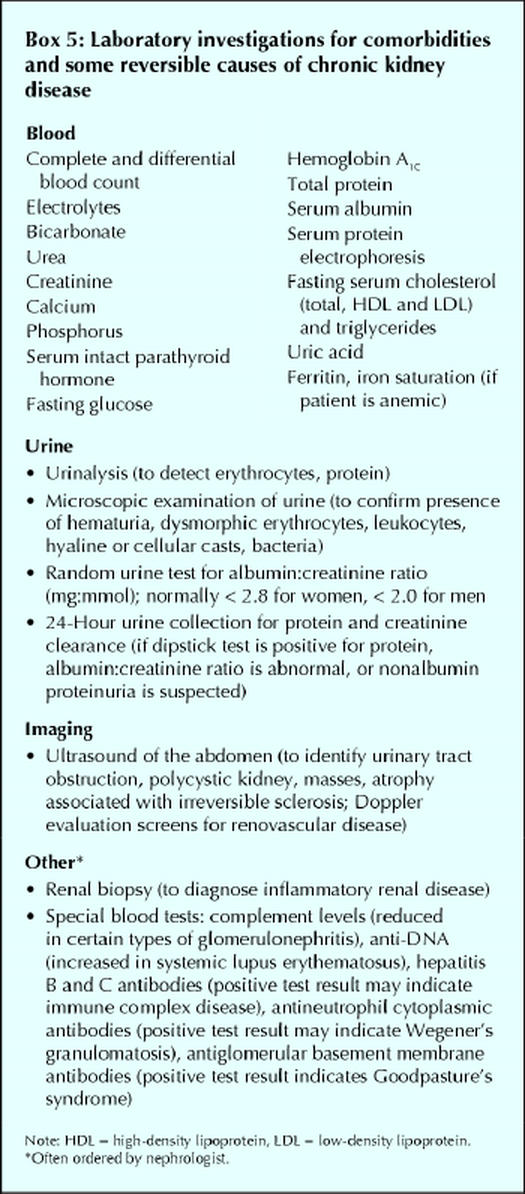
A focused history and physical examination of patients with suspected chronic kidney disease may be useful for identifying causes and sequelae of kidney disease (Boxes 2, 3 and 4). Several laboratory tests are also useful in the investigation of chronic kidney disease (Box 5), both to rule out comorbidities that can predispose patients to the disease and to monitor metabolic conditions that can develop as a result of chronic kidney disease. It is important to distinguish between acute and chronic renal failure. Features that support chronicity include previously elevated serum creatinine values, ultrasound evidence of small kidneys and the presence of metabolic abnormalities discussed in the next section. If there is uncertainty of the acuity of the renal failure, then immediate referral to a nephrologist is warranted.
Box 4.

Box 5.
Management
The following are steps in the management of patients with kidney disease.
1. Identify reversible causes of kidney disease
Reversible declines in kidney function are frequently due to volume contraction, urinary obstruction or toxic effects of medications. Medications that can cause a decline in kidney function include angiotensin-converting enzyme (ACE) inhibitors, angiotensin-receptor blockers (ARBs), NSAIDs, certain antibiotics (aminoglycosides and amphotericin B) and intravenous radiocontrast agents. If intravenously administered radiocontrast is to be used, oral acetylcysteine administration may attenuate the toxic effects.11 Kappel and Calissi,12 in part 3 of this series, reviewed safe drug prescribing for patients with renal insufficiency.
2. Identify kidney disease requiring disease-specific therapies
Causes of renal failure that may require specific therapies include renovascular disease, glomerulonephritis (including the nephrotic syndrome), hypercalcemia, multiple myeloma and upper urinary tract infections. Nephrologists should be involved in the care of patients with any of these diagnoses.
3. Identify and treat factors associated with progression of kidney disease
Hypertension
The recommended target blood pressure for patients with renal disease is less than 130/80 mm Hg in those with low levels of protein excretion and less than 125/75 mm Hg in those with high levels of protein excretion (≥ 1–3 g/d or more).13 The choice of antihypertensive agent is generally felt to be less important than the achievement of optimal blood pressure control, and patients often ultimately require 3 or more agents.14,15
ACE inhibitors are often used as first-line antihypertensive agents in patients with kidney disease because they not only decrease systemic blood pressure but also decrease intraglomerular blood pressure through a preferential dilatation of glomerular efferent arterioles. A number of large, randomized controlled trials have demonstrated the efficacy of ACE inhibitors in both the reduction of proteinuria and the slowing of renal disease progression.16,17,18 Patients taking ACE inhibitors should be monitored closely for the development of acute renal failure and hyperkalemia: serum creatinine and potassium levels should be checked 1 week and 1 month after starting therapy. Acute declines in renal function, defined as an increase in serum creatinine of 30% from baseline that stabilizes within the first 2 months of ACE inhibitor therapy, are tolerable, and in fact such declines have been associated with a slower progression of kidney disease after 3 or more years of ACE inhibitor therapy.19
Patients who cannot tolerate ACE inhibitors may be prescribed ARBs, which do not promote bradykinin accumulation and thus are not associated with the development of cough. Early studies have found that ARBs are as effective as ACE inhibitors in slowing the progression of renal disease.20
Diuretics are particularly useful in patients with extracellular fluid volume expansion. The choice of diuretic is determined by the glomerular filtration rate. Thiazides should be used in patients with a higher glomerular filtration rate (creatinine clearance > 50 mL/min [> 0.83 mL/s]) and more potent loop diuretics (e.g., furosemide) in patients with a lower glomerular filtration rate.15 Long-acting nondihydropyridine calcium-channel blockers (e.g., diltiazem) are recommended over the dihydropyridine subclass (e.g., amlodipine or nifedipine) because the former independently reduce cardiovascular mortality, proteinuria and progression of diabetic nephropathy.21,22 The dihydropyridine calcium-channel blockers, if prescribed without an ACE inhibitor, may result in increased proteinuria. When these agents are combined with ACE inhibitors, however, they reduce cardiovascular mortality, proteinuria and progressive nephropathy.13,23
Optimal antihypertensive combinations involve drugs with different mechanisms of activity. Effective combinations often include ACE inhibitors and diuretics, or diuretics and calcium-channel blockers. In addition, dietary sodium restriction is essential for controlling hypertension in patients with chronic kidney disease.
Proteinuria
Proteinuria is a hallmark of both diabetic and nondiabetic glomerular disease that is not only a marker of underlying renal disease but is also thought to contribute to declining kidney function.24 Proteinuria reduction is thus felt to be beneficial in patients with renal insufficiency. Effective strategies for reducing proteinuria include lowering intraglomerular pressure with ACE inhibitors, ARBs or nondihydropyridine calcium-channel blockers.15,25,26
Reports of 2 independent studies published in the last year demonstrated a significant reduction in the progression of type 2 diabetic nephropathy with the use of ARBs.27,28 Similar studies have not been performed with ACE inhibitors. However, the HOPE study demonstrated that there is a significant cardioprotective effect with the use of ACE inhibitors in patients with cardiac disease, including patients with diabetes.29 No explicit recommendations can be made about whether to choose an ACE inhibitor or an ARB for patients with the 3 indications for angiotension blockade: hypertension control, proteinuria reduction and cardioprotection. Combination therapy can be attempted if neither class sufficiently controls blood pressure or proteinuria independently; however, such therapy should be used cautiously, and serum potassium and creatinine levels should be checked 1 week and 1 month after starting the second medication, and after any dose change.
Hyperglycemia
For patients with diabetes, hyperglycemia contributes initially to the development of hyperfiltration and subsequently to sclerosis. The Diabetes Control and Complications Trial demonstrated that, among patients with type 1 diabetes, improved glycemic control both reduced the incidence of microvascular complications and delayed the progression of established microvascular complications.30 Thus, nutritional and medical interventions to optimize a patient's glycemic control are recommended. In addition, the United Kingdom Prospective Diabetes Study showed that intensive glucose control in patients with type 2 diabetes also reduces the incidence of microvascular complications, including nephropathy.31
4. Monitor and treat biochemical abnormalities
Anemia
Anemia is common in patients with chronic kidney disease. In those receiving dialysis it is associated with poor outcomes, including increased rates of hospital admission, coronary artery disease and left ventricular hypertrophy, and reduced quality of life.32,33,34 Anemia occurs because of a decrease in effective erythropoietin production and therefore often requires recombinant erythropoietin therapy. Often there is concomitant iron deficiency, due to poor oral intake and reduced intestinal iron absorption. Thus, adequate iron stores should be ensured before the initiation of erythropoietin therapy.35 Anemia treatment can significantly improve energy levels, sleep, cognitive function and health-related quality of life in patients receiving dialysis.36,37 Studies are underway to determine whether initiation of erythropoietin therapy in patients with moderate renal dysfunction prevents the development of left ventricular hypertrophy, other cardiovascular disease and death.
Mineral metabolism
Abnormalities in mineral metabolism affect calcium, phosphate and parathyroid hormone levels and occur early in renal disease. The underlying cause is the decreased ability of the kidney to excrete phosphate and to produce the active vitamin D metabolite (1,25-dihydroxyvitamin D), which leads to hypocalcemia and secondary hyperparathyroidism. Mineral metabolism abnormalities have been associated with renal osteodystrophy and calciphylaxis and, more recently, with cardiac and other vascular calcifications.38,39 Initial therapy includes restriction of dietary phosphate intake, often with the assistance of a renal dietitian, calcium supplementation with meals (to bind and prevent absorption of dietary phosphate from the intestine) and possibly oral vitamin D supplementation.
Dyslipidemia
Abnormalities in the serum lipid profile are common in patients with kidney disease and are characterized by elevated triglyceride levels, reduced total cholesterol, high-density lipoprotein (HDL) and low-density lipoprotein (LDL) levels, increased very-low-density and intermediate-density lipoproteins and triglyceride enrichment of LDL and HDL particles.40 In addition, comorbidities such as diabetes and atherosclerosis are independently associated with dyslipidemias. There is experimental animal evidence that cholesterol loading enhances glomerular injury41 and that pharmacological lowering of lipid levels slows the rate of progression of renal disease.42 However, to date, there is no clinical evidence as to the efficacy of lipid-lowering medications in slowing the progression of kidney disease. Because of the high prevalence of cardiovascular disease among patients with chronic kidney disease, it may be important to address high lipid levels. The National Cholesterol Education Program recommends reducing lipid levels in high-risk populations, including people with kidney disease.43 Cholesterol targets for lipid-lowering therapy are currently considered to be the same as those for the secondary prevention of cardiovascular disease.
Nutrition
Uremia causes a catabolic state and anorexia with decreased dietary protein intake,44 both of which lead to protein malnutrition. Protein-restricted diets may help reduce the rate of progression of kidney disease, but it is important to avoid protein malnutrition in this population. The assistance of renal dietitians is essential to balance the complex nutritional needs and restrictions (phosphate and protein restriction, with adequate protein and energy intake).
Metabolic acidosis
The inability of the kidney to regenerate sufficient bicarbonate to replace that consumed through the buffering of dietary acids can cause metabolic acidosis, which may in turn promote muscle catabolism45 and exacerbate metabolic bone disease.46 Sodium bicarbonate is effective for the treatment of metabolic acidosis, but it should be prescribed judiciously, owing to the risk of sodium loading and consequent volume expansion and hypertension. Bicarbonate therapy is typically considered when serum bicarbonate levels are less than 22 mmol/L; the usual starting dose is 500 mg twice daily. However, proper trials evaluating this strategy have not been undertaken.
Cardiovascular disease
Cardiovascular disease is present in up to 70% of patients commencing dialysis47 and is the leading cause of death in dialysis patients.1 Left ventricular hypertrophy, a risk factor for cardiovascular events, has been well documented in chronic kidney disease.48 In addition, patients with chronic kidney disease have a poor prognosis after myocardial infarction, with decreased survival and increased incidence of post-infarct complications (e.g., arrhythmias, heart failure, cardiogenic shock and acute mitral valve regurgitation) being more frequently observed with progressively worse kidney function.49 Traditional strategies to modify cardiac risk factors and prevent cardiovascular events should be implemented, but they are often neglected because of the misperception about the futility of care in this patient group.50
5. Refer patients to a nephrologist and renal care team
Consultation with a nephrologist is often desirable in the early stage of kidney disease in order to reduce the rate of progression of kidney insufficiency, to assist in optimizing treatment of comorbid conditions, to adjust medications affected by chronic kidney insufficiency and to help decide the appropriate timing of renal replacement therapy. Guidelines from the Canadian Society of Nephrology state that dialysis should be initiated when the creatinine clearance falls to 5–10 mL/min (0.08–0.17 mL/s) in asymptomatic patients or 15–20 mL/min (0.25–0.33 mL/s) in symptomatic patients.51 The society also advises that at least 1 year is required for a renal care team to prepare patients adequately for dialysis, including educating patients and their families about the different treatment modalities (transplantation, hemodialysis or peritoneal dialysis), and either planning for noncadaveric renal transplantation or creating peritoneal or vascular access.9 The Canadian Society of Nephrology guidelines recommend that an arteriovenous fistula be established when the creatinine clearance is 15–20 mL/min (0.25–0.33 mL/s).52
The case revisited
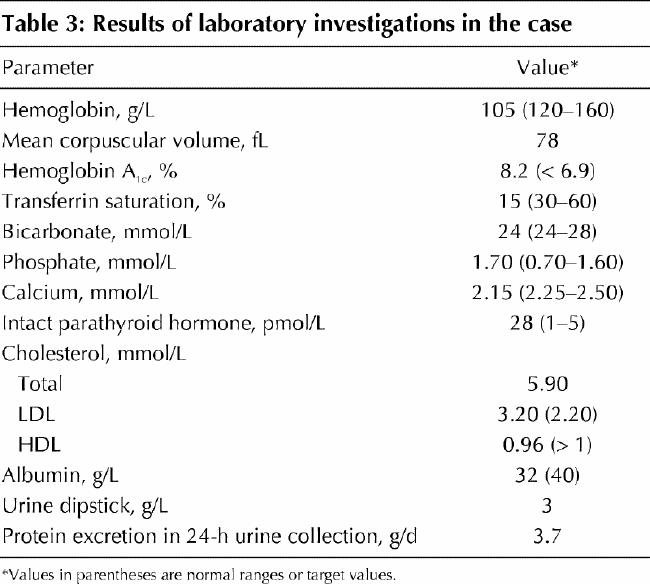
According to the Cockroft–Gault equation (Table 2), with a serum creatinine level of 140 μmol/L, weight of 55 kg and age of 60 years, the patient's creatinine clearance is calculated to be 33 mL/min (0.55 mL/s), which indicates that she has lost about 70% of her kidney function and has stage 3–4 chronic kidney disease. Further biochemical assessments are undertaken to assess for other risk factors and possible consequences of renal impairment (Table 3).
Table 3
A nephrologist is consulted, and an abdominal ultrasound is ordered, which shows no obstruction and bilaterally small echogenic kidneys. Results of serologic tests for hepatitis B and C and of serum protein electrophoresis are negative. The patient is judged to have kidney disease on the basis of her hypertension and diabetes. The relative contribution of each is not possible to determine from the information available, and a biopsy is judged unnecessary.
To reach the target blood pressure of 125/75 mm Hg, the thiazide diuretic therapy is stopped, ramipril therapy is started (with appropriate monitoring of serum electrolyte and creatinine levels), and diltiazam therapy is eventually added to her medication regimen. Six months after the initiation of the ACE inhibitor therapy, the patient's proteinuria is reduced, from 3.7 g/d to 1.8 g/d. Two months after cessation of hydrochlorothiazide therapy, her serum total cholesterol and LDL and HDL cholesterol levels are all still abnormal, and dietary fat restriction is attempted but proves ineffective. Atorvastatin therapy is initiated, and 4 months later her lipid profile returns to normal.
With nutritional counselling and continued use of subcutaneous insulin therapy, the patient's capillary blood glucose level (measured twice daily) improves, and the hemoglobin A1c is 6.8%. She is advised by a nutritionist about sodium and phosphate restriction, and calcium carbonate supplementation (1 tablet with each meal) is started. Six months later her serum calcium level has risen to 2.32 mmol/L, her serum phosphate level has decreased to 1.5 mmol/L, and her parathyroid hormone level has fallen to 15 (normally 1–5) pmol/L. Iron supplementation (ferrous sulfate 600 mg every night) results in the return of her iron stores to normal and the elevation of her hemoglobin concentration to 115 g/L, without the need for erythropoietin therapy. Potassium restriction is not required, but she is given a list of potassium-containing foods for possible future reference.
Discussions take place with the patient and her family regarding options for future renal replacement therapy, including living donor or cadaveric renal transplantation, and peritoneal dialysis or hemodialysis. The issue of timely creation of hemodialysis access or placement of a peritoneal dialysis catheter are also discussed.
Key points .
The serum creatinine level is an inaccurate marker of kidney function; it is more accurate to measure the creatinine clearance or, more easily, to use simple equations to calculate the creatinine clearance or the glomerular filtration rate (Table 2).
Once progressive kidney disease or a secondary cause of kidney disease that requires specific therapy is identified, the patient should be referred to a nephrologist and renal care team to develop a comprehensive plan for treatment and follow-up.
The target blood pressure for patients with kidney disease (< 130/80 mm Hg), and especially for those with proteinuria (< 125/75 mm Hg), is lower than that in the general population.
Hypertension and proteinuria are the 2 most important modifiable risk factors for patients with progressive kidney disease.
Multidisciplinary clinics, through educational and care programs, can assist patients and their families with delaying progression of kidney disease as well as planning for kidney replacement therapy and easing the transition to dialysis or transplantation.
CMAJ's nephrology series .
This series is now complete.
Morton AR, Iliescu EA, Wilson JWL. Nephrology: 1. Investigation and treatment of recurrent kidney stones. CMAJ 2002;166(2):213-8.
House AA, Cattran DC. Nephrology: 2. Evaluation of asymptomatic hematuria and proteinuria in adult primary care. CMAJ 2002;166(3):348-53.
Kappel J, Calissi P. Nephrology: 3. Safe drug prescribing for patients with renal insufficiency. CMAJ 2002;166 (4):473-7.
Stigant C, Stevens L, Levin A. Nephrology: 4. Strategies for the care of adults with chronic kidney disease. CMAJ 2003;168(12):1553-60.
Footnotes
This article has been peer reviewed.
Contributors: Drs. Stigant and Stevens contributed to the acquisition of references, the analysis and interpretation of data and the writing of the draft. Dr. Levin was responsible for the conception, design and writing of the draft. All authors approved of the final version.
Competing interests: None declared.
Correspondence to: Dr. Adeera Levin, Director, Kidney Function Clinic, St. Paul's Hospital, University of British Columbia, Rm. 6010-A, 1081 Burrard St., Vancouver BC V6Z 1Y6; fax 604 806-8120; alevin@providencehealth.bc.ca
References
- 1.Canadian Organ Replacement Register. Annual report 2002. Ottawa: Canadian Institute for Health Information; 2002.
- 2.K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Kidney Disease Outcome Quality Initiative. Am J Kidney Dis 2002;39(Suppl 2):S1-246. [PubMed]
- 3.McCullough PA, Soman SS, Shah SS, Smith ST, Marks KR, Yee J, Borzak S. Risks associated with renal dysfunction in patients in the coronary care unit. J Am Coll Cardiol 2000;36(3):679-84. [DOI] [PubMed]
- 4.Curtis B, Barrett BJ, Levin A. Identifying and slowing progressive chronic renal failure. Can Fam Physician 2001;47:2512-8. [PMC free article] [PubMed]
- 5.Ifudu O, Dawood M, Homel P, Friedman EA. Excess morbidity in patients starting uremia therapy without prior care by a nephrologist. Am J Kidney Dis 1996; 28(6):841-5. [DOI] [PubMed]
- 6.Levin A, Lewis M, Mortiboy P, Faber S, Hare I, Porter EC, et al. Multidisciplinary predialysis programs: quantification and limitations of their impact on patient outcomes in two Canadian settings. Am J Kidney Dis 1997;29(4): 533-40. [DOI] [PubMed]
- 7.USRDS 1998 annual report. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health, US Department of Health and Human Services; 1998.
- 8.Gorriz JL, Sancho A, Pallardo LM, Amoedo ML, Barril G, Salgueira M, et al. Longer pre-dialysis nephrological care is associated with improved long-term survival of dialysis patients. More facts. Nephrol Dial Transplant 2002;17(7): 1354-5. [DOI] [PubMed]
- 9.Mendelssohn DC, Barrett BJ, Brownscombe LM, Ethier J, Greenberg DE, Kanani SD, et al. Elevated levels of serum creatinine: recommendations for management and referral. CMAJ 1999;161(4):413-7. [PMC free article] [PubMed]
- 10.Cockroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron 1976;16(1):31-41. [DOI] [PubMed]
- 11.Tepel M, van der Giet M, Schwarzfeld C, Laufer U, Liermann D, Zidek W. Prevention of radiographic-contrast-agent-induced reductions in renal function by acetylcysteine. N Engl J Med 2000;343(3):180-4. [DOI] [PubMed]
- 12.Kappel J, Calissi P. Nephrology: 3. Safe drug prescribing for patients with renal insufficiency. CMAJ 2002;166(4):473-7. [PMC free article] [PubMed]
- 13.Feldman RD, Campbell N, Larochelle P, Bolli P, Burgess ED, Carruthers SG, et al. 1999 Canadian recommendations for the management of hypertension. Task Force for the Development of the 1999 Canadian Recommendations for the Management of Hypertension. CMAJ 1999;161(Suppl 12):S1-17. [PMC free article] [PubMed]
- 14.Hansson L, Zanchetti A, Carruthers SG, Dahlof B, Elmfeldt D, Julius S, et al. Effects of intensive blood-pressure lowering and low-dose aspirin in patients with hypertension: principal results of the Hypertension Optimal Treatment (HOT) randomised trial. HOT Study Group. Lancet 1998;351:1755-62. [DOI] [PubMed]
- 15.Bakris GL, Williams M, Dworkin L, Elliott WJ, Epstein M, Toto R, et al. Preserving renal function in adults with hypertension and diabetes: a consensus approach. National Kidney Foundation Hypertension and Diabetes Executive Committees Working Group. Am J Kidney Dis 2000;36(3):646-61. [DOI] [PubMed]
- 16.Ruggenenti P, Perna A, Gherardi G, Garini G, Zoccali C, Salvadori M, et al. Renoprotective properties of ACE-inhibition in non-diabetic nephropathies with non-nephrotic proteinuria. Lancet 1999;354:359-64. [DOI] [PubMed]
- 17.Ruggenenti P, Perna A, Gherardi G, Gaspari F, Benini R, Remuzzi G. Renal function and requirement for dialysis in chronic nephropathy patients on long-term ramipril: REIN follow-up trial. Gruppo Italiano di Studi Epidemiologici in Nefrologia (GISEN). Ramipril efficacy in nephropathy. Lancet 1998;352:1252-6. [DOI] [PubMed]
- 18.Maschio G, Alberti D, Janin G, Locatelli F, Mann JF, Motolese M, et al. Effect of the angiotensin-converting-enzyme inhibitor benazepril on the progression of chronic renal insufficiency. The Angiotensin-Converting-Enzyme Inhibition in Progressive Renal Insufficiency Study Group. N Engl J Med 1996; 334 (15):939-45. [DOI] [PubMed]
- 19.Bakris GL, Weir MR. Angiotensin-converting enzyme inhibitor-associated elevations in serum creatinine: Is this a cause for concern? Arch Intern Med 2000; 160(5):685-93. [DOI] [PubMed]
- 20.Kakinuma Y, Kawamura T, Bills T, Yoshioka T, Ichikawa I, Fogo A. Blood pressure-independent effect of angiotensin inhibition on vascular lesions of chronic renal failure. Kidney Int 1992;42(1):46-55. [DOI] [PubMed]
- 21.Bakris GL, Copley JB, Vicknair N, Sadler R, Leurgans S. Calcium channel blockers versus other antihypertensive therapies on progression of NIDDM associated nephropathy. Kidney Int 1996;50(5):1641-50. [DOI] [PubMed]
- 22.Effect of verapamil on mortality and major events after acute myocardial infarction (the Danish Verapamil Infarction Trial II — DAVIT II). Am J Cardiol 1990;66(10):779-85. [DOI] [PubMed]
- 23.Tatti P, Pahor M, Byington RP, Di Mauro P, Guarisco R, Strollo G, et al. Outcome results of the Fosinopril Versus Amlodipine Cardiovascular Events Randomized Trial (FACET) in patients with hypertension and NIDDM. Diabetes Care 1998;21(4):597-603. [DOI] [PubMed]
- 24.Remuzzi G, Ruggenenti P, Benigni A. Understanding the nature of renal disease progression. Kidney Int 1997;51(1):2-15. [DOI] [PubMed]
- 25.Lewis EJ, Hunsicker LG, Bain RP, Rohde RD. The effect of angiotensin-converting-enzyme inhibition on diabetic nephropathy. The Collaborative Study Group. N Engl J Med 1993;329(20):1456-62. [DOI] [PubMed]
- 26.Ravid M, Savin H, Jutrin I, Bental T, Katz B, Lishner M. Long-term stabilizing effect of angiotensin-converting enzyme inhibition on plasma creatinine and on proteinuria in normotensive type II diabetic patients. Ann Intern Med 1993;118(8):577-81. [DOI] [PubMed]
- 27.Parving HH, Lehnert H, Brochner-Mortensen J, Gomis R, Andersen S, Arner P. The effect of irbesartan on the development of diabetic nephropathy in patients with type 2 diabetes. N Engl J Med 2001;345(12):870-8. [DOI] [PubMed]
- 28.Brenner BM, Cooper ME, de Zeeuw D, Keane WF, Mitch WE, Parving HH, et al. Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med 2001;345(12):861-9. [DOI] [PubMed]
- 29.Yusuf S, Sleight P, Pogue J, Bosch J, Davies R, Dagenais G. Effects of an angiotensin-converting-enzyme inhibitor, ramipril, on cardiovascular events in high-risk patients. Heart Outcomes Prevention Evaluation Study Investigators. N Engl J Med 2000;342(3):145-53. [DOI] [PubMed]
- 30.The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. Diabetes Control and Complications Trial Research Group. N Engl J Med 1993;329(14):977-86. [DOI] [PubMed]
- 31.Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). UK Prospective Diabetes Study (UKPDS) Group. Lancet 1998;352:837-53. [PubMed]
- 32.Collins AJ, Ma JZ, Xia A, Ebben J. Trends in anemia treatment with erythropoietin usage and patient outcomes. Am J Kidney Dis 1998;32(6 Suppl 4):S133-41. [DOI] [PubMed]
- 33.Levin A. Anaemia in the patient with renal insufficiency: documenting the impact and reviewing treatment strategies. Nephrol Dial Transplant 1999;14(2):292-5. [DOI] [PubMed]
- 34.Powe NR, Griffiths RI, Watson AJ, Anderson GF, de Lissovoy G, Greer JW, et al. Effect of recombinant erythropoietin on hospital admissions, readmissions, length of stay, and costs of dialysis patients. J Am Soc Nephrol 1994;4(7):1455-65. [DOI] [PubMed]
- 35.Goch J, Birgegard G, Danielson BG, Wikstrom B. Iron absorption in patients with chronic uremia on maintenance hemodialysis and in healthy volunteers measured with a simple oral iron load test. Nephron 1996;73(3):403-6. [DOI] [PubMed]
- 36.Benz RL, Pressman MR, Hovick ET, Peterson DD. A preliminary study of the effects of correction of anemia with recombinant human erythropoietin therapy on sleep, sleep disorders, and daytime sleepiness in hemodialysis patients (The SLEEPO study). Am J Kidney Dis 1999;34(6):1089-95. [DOI] [PubMed]
- 37.Pickett JL, Theberge DC, Brown WS, Schweitzer SU, Nissenson AR. Normalizing hematocrit in dialysis patients improves brain function. Am J Kidney Dis 1999;33(6):1122-30. [DOI] [PubMed]
- 38.Goodman WG, Goldin J, Kuizon BD, Yoon C, Gales B, Sider D, et al. Coronary-artery calcification in young adults with end-stage renal disease who are undergoing dialysis. N Engl J Med 2000;342(20):1478-83. [DOI] [PubMed]
- 39.Block GA, Port FK. Re-evaluation of risks associated with hyperphosphatemia and hyperparathyroidism in dialysis patients: recommendations for a change in management. Am J Kidney Dis 2000;35(6):1226-37. [DOI] [PubMed]
- 40.Shoji T, Ishimura E, Inaba M, Tabata T, Nishizawa Y. Atherogenic lipoproteins in end-stage renal disease. Am J KidneyDis 2001;38(4 Suppl 1):S30-3. [DOI] [PubMed]
- 41.Diamond JR, Karnovsky MJ. Exacerbation of chronic aminonucleoside nephrosis by dietary cholesterol supplementation. Kidney Int 1987;32(5):671-7. [DOI] [PubMed]
- 42.Fried LF, Orchard TJ, Kasiske BL. Effect of lipid reduction on the progression of renal disease: a meta-analysis. Kidney Int 2001;59(1):260-9. [DOI] [PubMed]
- 43.Summary of the second report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel II). JAMA 1993;269(23):3015-23. [PubMed]
- 44.Ikizler TA, Greene JH, Wingard RL, Parker RA, Hakim RM. Spontaneous dietary protein intake during progression of chronic renal failure. J Am Soc Nephrol 1995;6(5):1386-91. [DOI] [PubMed]
- 45.Williams B, Hattersley J, Layward E, Walls J. Metabolic acidosis and skeletal muscle adaptation to low protein diets in chronic uremia. Kidney Int 1991; 40(4):779-86. [DOI] [PubMed]
- 46.Bushinsky DA. The contribution of acidosis to renal osteodystrophy. Kidney Int 1995;47(6):1816-32. [DOI] [PubMed]
- 47.Foley RN, Parfrey PS, Harnett JD, Kent GM, Martin CJ, Murray DC, et al. Clinical and echocardiographic disease in patients starting end-stage renal disease therapy. Kidney Int 1995;47(1):186-92. [DOI] [PubMed]
- 48.Levin A, Singer J, Thompson CR, Ross H, Lewis M. Prevalent left ventricular hypertrophy in the predialysis population: identifying opportunities for intervention. Am J Kidney Dis 1996;27(3):347-54. [DOI] [PubMed]
- 49.Beattie JN, Soman SS, Sandberg KR, Yee J, Borzak S, Garg M, et al. Determinants of mortality after myocardial infarction in patients with advanced renal dysfunction. Am J Kidney Dis 2001;37(6):1191-200. [DOI] [PubMed]
- 50.Chuahirun T, Wesson DE. Cigarette smoking predicts faster progression of type 2 established diabetic nephropathy despite ACE inhibition. Am J Kidney Dis 2002;39(2):376-82. [DOI] [PubMed]
- 51.Churchill DN, Blake PG, Jindal KK, Toffelmire EB, Goldstein MB. Clinical practice guidelines for initiation of dialysis. Canadian Society of Nephrology. J Am Soc Nephrol 1999;10(Suppl 13):S289-91. [PubMed]
- 52.Jindal KK, Ethier JH, Lindsay RM, Barre PE, Kappel JE, Carlisle EJF, et al. Clinical practice guidelines for vascular access. Canadian Society of Nephrology. J Am Soc Nephrol 1999;10(Suppl 13):S297-305. [PubMed]


