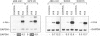Abstract
Oxidant carcinogens interact with multiple cellular targets including membranes, proteins, and nucleic acids. They cause structural damage to DNA and have the potential to mutate cancer-related genes. At the same time, oxidants activate signal transduction pathways and alter the expression of growth- and differentiation-related genes. Indeed, the carcinogenic action of oxidants results from the superposition of these genetic and epigenetic effects. All cells possess elaborate antioxidant defense systems that consist of interacting low and high molecular weight components. Among them, superoxide dismutases (SOD), glutathione peroxidases (GPx), and catalase (CAT) play a central role. Our studies with mouse epidermal cells demonstrate that the balance between several antioxidant enzymes rather than the activity of a single component determines the degree of protection. Unexpectedly, increased levels of Cu,Zn-SOD alone in stable transfectants resulted in sensitization to oxidative chromosomal aberrations and DNA strand breaks. However, a concomitant increase in CAT or GPx in double transfectants corrected or overcorrected the hypersensitivity of the SOD clones depending on the ratios of activities CAT/SOD or GPx/SOD. The cellular antioxidant capacity also affected oxidant induction of the growth-related immediate early protooncogene c-fos. Increases in CAT or SOD reduced the accumulation of c-fos message, albeit for different reasons. The cellular antioxidant defense also affects the action of UVB light (290-320 nm) that represents the most potent carcinogenic wavelength range of the solar spectrum. UVB light is known to exert its action in part through oxidative mechanisms. Increases in CAT and GPx protected mouse epidermal cells from UVB-induced DNA breakage.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alexander-Williams J. Inflammatory disease of the bowel: the risk of cancer. Dis Colon Rectum. 1976 Oct;19(7):579–581. doi: 10.1007/BF02590970. [DOI] [PubMed] [Google Scholar]
- Amstad P. A., Krupitza G., Cerutti P. A. Mechanism of c-fos induction by active oxygen. Cancer Res. 1992 Jul 15;52(14):3952–3960. [PubMed] [Google Scholar]
- Amstad P., Cerutti P. Genetic modulation of the cellular antioxidant defense capacity. Environ Health Perspect. 1990 Aug;88:77–82. doi: 10.1289/ehp.908877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amstad P., Peskin A., Shah G., Mirault M. E., Moret R., Zbinden I., Cerutti P. The balance between Cu,Zn-superoxide dismutase and catalase affects the sensitivity of mouse epidermal cells to oxidative stress. Biochemistry. 1991 Sep 24;30(38):9305–9313. doi: 10.1021/bi00102a024. [DOI] [PubMed] [Google Scholar]
- Argyris T. S., Slaga T. J. Promotion of carcinomas by repeated abrasion in initiated skin of mice. Cancer Res. 1981 Dec;41(12 Pt 1):5193–5195. [PubMed] [Google Scholar]
- Badwey J. A., Karnovsky M. L. Active oxygen species and the functions of phagocytic leukocytes. Annu Rev Biochem. 1980;49:695–726. doi: 10.1146/annurev.bi.49.070180.003403. [DOI] [PubMed] [Google Scholar]
- Bloch C. A., Ausubel F. M. Paraquat-mediated selection for mutations in the manganese-superoxide dismutase gene sodA. J Bacteriol. 1986 Nov;168(2):795–798. doi: 10.1128/jb.168.2.795-798.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bos J. L. ras oncogenes in human cancer: a review. Cancer Res. 1989 Sep 1;49(17):4682–4689. [PubMed] [Google Scholar]
- Cantoni O., Sestili P., Cattabeni F., Bellomo G., Pou S., Cohen M., Cerutti P. Calcium chelator Quin 2 prevents hydrogen-peroxide-induced DNA breakage and cytotoxicity. Eur J Biochem. 1989 Jun 15;182(2):209–212. doi: 10.1111/j.1432-1033.1989.tb14819.x. [DOI] [PubMed] [Google Scholar]
- Caron de Fromentel C., Soussi T. TP53 tumor suppressor gene: a model for investigating human mutagenesis. Genes Chromosomes Cancer. 1992 Jan;4(1):1–15. doi: 10.1002/gcc.2870040102. [DOI] [PubMed] [Google Scholar]
- Cerutti P. A. Prooxidant states and tumor promotion. Science. 1985 Jan 25;227(4685):375–381. doi: 10.1126/science.2981433. [DOI] [PubMed] [Google Scholar]
- Cerutti P. A. Response modification creates promotability in multistage carcinogenesis. Carcinogenesis. 1988 Apr;9(4):519–526. doi: 10.1093/carcin/9.4.519. [DOI] [PubMed] [Google Scholar]
- Cerutti P. A., Trump B. F. Inflammation and oxidative stress in carcinogenesis. Cancer Cells. 1991 Jan;3(1):1–7. [PubMed] [Google Scholar]
- Chiocca S. M., Sandy M. S., Cerutti P. A. Genotypic analysis of N-ethyl-N-nitrosourea-induced mutations by Taq I restriction fragment length polymorphism/polymerase chain reaction in the c-H-ras1 gene. Proc Natl Acad Sci U S A. 1992 Jun 15;89(12):5331–5335. doi: 10.1073/pnas.89.12.5331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coohill T. P., Peak M. J., Peak J. G. The effects of the ultraviolet wavelengths of radiation present in sunlight on human cells in vitro. Photochem Photobiol. 1987 Dec;46(6):1043–1050. doi: 10.1111/j.1751-1097.1987.tb04891.x. [DOI] [PubMed] [Google Scholar]
- Crawford D. R., Amstad P. A., Foo D. D., Cerutti P. A. Constitutive and phorbol-myristate-acetate regulated antioxidant defense of mouse epidermal JB6 cells. Mol Carcinog. 1989;2(3):136–143. doi: 10.1002/mc.2940020306. [DOI] [PubMed] [Google Scholar]
- Devary Y., Gottlieb R. A., Lau L. F., Karin M. Rapid and preferential activation of the c-jun gene during the mammalian UV response. Mol Cell Biol. 1991 May;11(5):2804–2811. doi: 10.1128/mcb.11.5.2804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devary Y., Gottlieb R. A., Smeal T., Karin M. The mammalian ultraviolet response is triggered by activation of Src tyrosine kinases. Cell. 1992 Dec 24;71(7):1081–1091. doi: 10.1016/s0092-8674(05)80058-3. [DOI] [PubMed] [Google Scholar]
- Elroy-Stein O., Bernstein Y., Groner Y. Overproduction of human Cu/Zn-superoxide dismutase in transfected cells: extenuation of paraquat-mediated cytotoxicity and enhancement of lipid peroxidation. EMBO J. 1986 Mar;5(3):615–622. doi: 10.1002/j.1460-2075.1986.tb04255.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein J. H. Photocarcinogenesis: a review. Natl Cancer Inst Monogr. 1978 Dec;(50):13–25. [PubMed] [Google Scholar]
- Feng J. L., Villeponteau B. Serum stimulation of the c-fos enhancer induces reversible changes in c-fos chromatin structure. Mol Cell Biol. 1990 Mar;10(3):1126–1133. doi: 10.1128/mcb.10.3.1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng J., Villeponteau B. High-resolution analysis of c-fos chromatin accessibility using a novel DNase I-PCR assay. Biochim Biophys Acta. 1992 Apr 6;1130(3):253–258. doi: 10.1016/0167-4781(92)90437-5. [DOI] [PubMed] [Google Scholar]
- Feret E., Larouze B., Diop B., Sow M., London W. T., Blumberg B. S. Epidemiology of hepatitis B virus infection in the rural community of Tip, Senegal. Am J Epidemiol. 1987 Jan;125(1):140–149. doi: 10.1093/oxfordjournals.aje.a114497. [DOI] [PubMed] [Google Scholar]
- Forbes P. D., Davies R. E., Urbach F. Experimental ultraviolet photocarcinogenesis: wavelength interactions and time-dose relationships. Natl Cancer Inst Monogr. 1978 Dec;(50):31–38. [PubMed] [Google Scholar]
- Gey K. F., Brubacher G. B., Stähelin H. B. Plasma levels of antioxidant vitamins in relation to ischemic heart disease and cancer. Am J Clin Nutr. 1987 May;45(5 Suppl):1368–1377. doi: 10.1093/ajcn/45.5.1368. [DOI] [PubMed] [Google Scholar]
- Ghosh R., Amstad P., Cerutti P. UVB-induced DNA breaks interfere with transcriptional induction of c-fos. Mol Cell Biol. 1993 Nov;13(11):6992–6999. doi: 10.1128/mcb.13.11.6992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gindhart T. D., Nakamura Y., Stevens L. A., Hegameyer G. A., West M. W., Smith B. M., Colburn N. H. Genes and signal transduction in tumor promotion: conclusions from studies with promoter resistant variants of JB-6 mouse epidermal cells. Carcinog Compr Surv. 1985;8:341–367. [PubMed] [Google Scholar]
- Groner Y., Avraham K. B., Schickler M., Yarom R., Knobler H. Clinical symptoms of Down syndrome are manifested in transgenic mice overexpressing the human Cu/Zn-superoxide dismutase gene. Prog Clin Biol Res. 1990;360:233–262. [PubMed] [Google Scholar]
- Hariharan P. V., Cerutti P. A. Formation of products of the 5,6-dihydroxydihydrothymine type by ultraviolet light in HeLa cells. Biochemistry. 1977 Jun 14;16(12):2791–2795. doi: 10.1021/bi00631a032. [DOI] [PubMed] [Google Scholar]
- Hirschi M., Netrawali M. S., Remsen J. F., Cerutti P. A. Formation of DNA single-strand breaks by near-ultraviolet and gamma-rays in normal and Bloom's syndrome skin fibroblasts. Cancer Res. 1981 May;41(5):2003–2007. [PubMed] [Google Scholar]
- Hodgson E. K., Fridovich I. The interaction of bovine erythrocyte superoxide dismutase with hydrogen peroxide: chemiluminescence and peroxidation. Biochemistry. 1975 Dec 2;14(24):5299–5303. doi: 10.1021/bi00695a011. [DOI] [PubMed] [Google Scholar]
- Hollstein M., Sidransky D., Vogelstein B., Harris C. C. p53 mutations in human cancers. Science. 1991 Jul 5;253(5015):49–53. doi: 10.1126/science.1905840. [DOI] [PubMed] [Google Scholar]
- Kedziora J., Bartosz G. Down's syndrome: a pathology involving the lack of balance of reactive oxygen species. Free Radic Biol Med. 1988;4(5):317–330. doi: 10.1016/0891-5849(88)90052-4. [DOI] [PubMed] [Google Scholar]
- Kensler T. W., Bush D. M., Kozumbo W. J. Inhibition of tumor promotion by a biomimetic superoxide dismutase. Science. 1983 Jul 1;221(4605):75–77. doi: 10.1126/science.6857269. [DOI] [PubMed] [Google Scholar]
- Kono Y., Fridovich I. Superoxide radical inhibits catalase. J Biol Chem. 1982 May 25;257(10):5751–5754. [PubMed] [Google Scholar]
- Kozumbo W. J., Trush M. A., Kensler T. W. Are free radicals involved in tumor promotion? Chem Biol Interact. 1985 Jul;54(2):199–207. doi: 10.1016/s0009-2797(85)80163-0. [DOI] [PubMed] [Google Scholar]
- Larsson R., Cerutti P. Oxidants induce phosphorylation of ribosomal protein S6. J Biol Chem. 1988 Nov 25;263(33):17452–17458. [PubMed] [Google Scholar]
- Larsson R., Cerutti P. Translocation and enhancement of phosphotransferase activity of protein kinase C following exposure in mouse epidermal cells to oxidants. Cancer Res. 1989 Oct 15;49(20):5627–5632. [PubMed] [Google Scholar]
- Lewis J. G., Hamilton T., Adams D. O. The effect of macrophage development on the release of reactive oxygen intermediates and lipid oxidation products, and their ability to induce oxidative DNA damage in mammalian cells. Carcinogenesis. 1986 May;7(5):813–818. doi: 10.1093/carcin/7.5.813. [DOI] [PubMed] [Google Scholar]
- Mao G. D., Thomas P. D., Lopaschuk G. D., Poznansky M. J. Superoxide dismutase (SOD)-catalase conjugates. Role of hydrogen peroxide and the Fenton reaction in SOD toxicity. J Biol Chem. 1993 Jan 5;268(1):416–420. [PubMed] [Google Scholar]
- Menkes M. S., Comstock G. W., Vuilleumier J. P., Helsing K. J., Rider A. A., Brookmeyer R. Serum beta-carotene, vitamins A and E, selenium, and the risk of lung cancer. N Engl J Med. 1986 Nov 13;315(20):1250–1254. doi: 10.1056/NEJM198611133152003. [DOI] [PubMed] [Google Scholar]
- Mirault M. E., Tremblay A., Beaudoin N., Tremblay M. Overexpression of seleno-glutathione peroxidase by gene transfer enhances the resistance of T47D human breast cells to clastogenic oxidants. J Biol Chem. 1991 Nov 5;266(31):20752–20760. [PubMed] [Google Scholar]
- Muehlematter D., Larsson R., Cerutti P. Active oxygen induced DNA strand breakage and poly ADP-ribosylation in promotable and non-promotable JB6 mouse epidermal cells. Carcinogenesis. 1988 Feb;9(2):239–245. doi: 10.1093/carcin/9.2.239. [DOI] [PubMed] [Google Scholar]
- Nakamura Y., Gindhart T. D., Winterstein D., Tomita I., Seed J. L., Colburn N. H. Early superoxide dismutase-sensitive event promotes neoplastic transformation in mouse epidermal JB6 cells. Carcinogenesis. 1988 Feb;9(2):203–207. doi: 10.1093/carcin/9.2.203. [DOI] [PubMed] [Google Scholar]
- Niggli H. J., Cerutti P. A. Cyclobutane-type pyrimidine photodimer formation and excision in human skin fibroblasts after irradiation with 313-nm ultraviolet light. Biochemistry. 1983 Mar 15;22(6):1390–1395. doi: 10.1021/bi00275a011. [DOI] [PubMed] [Google Scholar]
- O'Connell J. F., Klein-Szanto A. J., DiGiovanni D. M., Fries J. W., Slaga T. J. Enhanced malignant progression of mouse skin tumors by the free-radical generator benzoyl peroxide. Cancer Res. 1986 Jun;46(6):2863–2865. [PubMed] [Google Scholar]
- Ochi T., Cerutti P. A. Clastogenic action of hydroperoxy-5,8,11,13-icosatetraenoic acids on the mouse embryo fibroblasts C3H/10T1/2. Proc Natl Acad Sci U S A. 1987 Feb;84(4):990–994. doi: 10.1073/pnas.84.4.990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omar B. A., McCord J. M. The cardioprotective effect of Mn-superoxide dismutase is lost at high doses in the postischemic isolated rabbit heart. Free Radic Biol Med. 1990;9(6):473–478. doi: 10.1016/0891-5849(90)90124-2. [DOI] [PubMed] [Google Scholar]
- Pourzand C., Cerutti P. Genotypic mutation analysis by RFLP/PCR. Mutat Res. 1993 Jul;288(1):113–121. doi: 10.1016/0027-5107(93)90213-y. [DOI] [PubMed] [Google Scholar]
- Radler-Pohl A., Sachsenmaier C., Gebel S., Auer H. P., Bruder J. T., Rapp U., Angel P., Rahmsdorf H. J., Herrlich P. UV-induced activation of AP-1 involves obligatory extranuclear steps including Raf-1 kinase. EMBO J. 1993 Mar;12(3):1005–1012. doi: 10.1002/j.1460-2075.1993.tb05741.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronai Z. A., Okin E., Weinstein I. B. Ultraviolet light induces the expression of oncogenes in rat fibroblast and human keratinocyte cells. Oncogene. 1988 Feb;2(2):201–204. [PubMed] [Google Scholar]
- Rosen D. R., Siddique T., Patterson D., Figlewicz D. A., Sapp P., Hentati A., Donaldson D., Goto J., O'Regan J. P., Deng H. X. Mutations in Cu/Zn superoxide dismutase gene are associated with familial amyotrophic lateral sclerosis. Nature. 1993 Mar 4;362(6415):59–62. doi: 10.1038/362059a0. [DOI] [PubMed] [Google Scholar]
- Scott M. D., Meshnick S. R., Eaton J. W. Superoxide dismutase-rich bacteria. Paradoxical increase in oxidant toxicity. J Biol Chem. 1987 Mar 15;262(8):3640–3645. [PubMed] [Google Scholar]
- Shah G., Ghosh R., Amstad P. A., Cerutti P. A. Mechanism of induction of c-fos by ultraviolet B (290-320 nm) in mouse JB6 epidermal cells. Cancer Res. 1993 Jan 1;53(1):38–45. [PubMed] [Google Scholar]
- Sinet P. M., Lejeune J., Jerome H. Trisomy 21 (Down's syndrome). Glutathione peroxidase, hexose monophoshate shunt and I.Q. Life Sci. 1979 Jan 1;24(1):29–33. doi: 10.1016/0024-3205(79)90276-5. [DOI] [PubMed] [Google Scholar]
- Stalder J., Groudine M., Dodgson J. B., Engel J. D., Weintraub H. Hb switching in chickens. Cell. 1980 Apr;19(4):973–980. doi: 10.1016/0092-8674(80)90088-4. [DOI] [PubMed] [Google Scholar]
- Sun Y. Free radicals, antioxidant enzymes, and carcinogenesis. Free Radic Biol Med. 1990;8(6):583–599. doi: 10.1016/0891-5849(90)90156-d. [DOI] [PubMed] [Google Scholar]
- Viaje A., Slaga T. J., Wigler M., Weinstein I. B. Effects of antiinflammatory agents on mouse skin tumor promotion, epidermal DNA synthesis, phorbol ester-induced cellular proliferation, and production of plasminogen activator. Cancer Res. 1977 May;37(5):1530–1536. [PubMed] [Google Scholar]
- Weintraub H. Assembly and propagation of repressed and depressed chromosomal states. Cell. 1985 Oct;42(3):705–711. doi: 10.1016/0092-8674(85)90267-3. [DOI] [PubMed] [Google Scholar]
- Weitzman S. A., Weitberg A. B., Clark E. P., Stossel T. P. Phagocytes as carcinogens: malignant transformation produced by human neutrophils. Science. 1985 Mar 8;227(4691):1231–1233. doi: 10.1126/science.3975611. [DOI] [PubMed] [Google Scholar]
- Zimmerman R., Cerutti P. Active oxygen acts as a promoter of transformation in mouse embryo C3H/10T1/2/C18 fibroblasts. Proc Natl Acad Sci U S A. 1984 Apr;81(7):2085–2087. doi: 10.1073/pnas.81.7.2085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerman R., Cerutti P. Active oxygen acts as a promoter of transformation in mouse embryo C3H/10T1/2/C18 fibroblasts. Proc Natl Acad Sci U S A. 1984 Apr;81(7):2085–2087. doi: 10.1073/pnas.81.7.2085. [DOI] [PMC free article] [PubMed] [Google Scholar]