Abstract
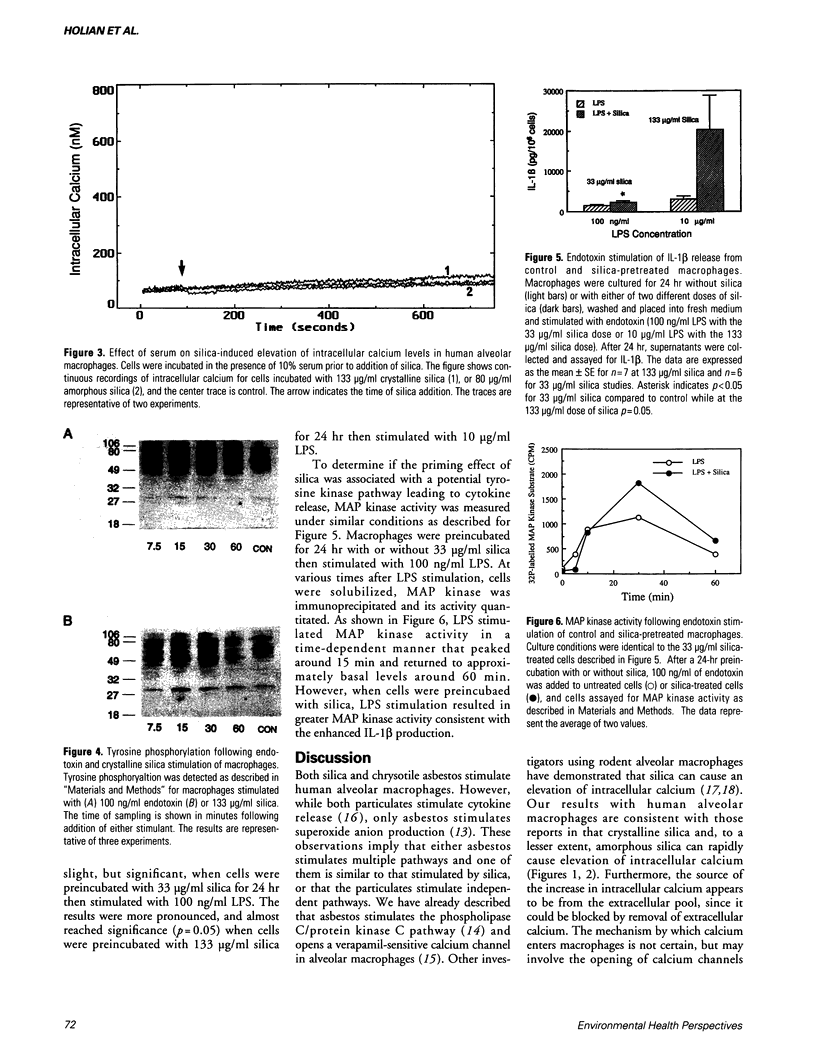
Asbestos and silica are well-known fibrogenic dusts. However, there is no comprehensive understanding of the molecular and cellular events that lead to fibrosis as a consequence of asbestos or silica inhalation. Previous studies have shown that asbestos stimulates superoxide anion production in alveolar macrophages through the phospholipase C/protein kinase C pathway. In contrast, silica does not appear to activate this pathway nor stimulate superoxide anion production, but silica does stimulate cytokine release by some undetermined pathway. Therefore, using human alveolar macrophages isolated from normal healthy volunteers, we evaluated the potential involvement of intracellular calcium and tyrosine kinases as potential signal transduction pathways. In the absence of serum, crystalline silica, and to a lesser extent amorphous silica, caused a rapid and dose-dependent elevation of intracellular calcium coming from the extracellular space. However, in the presence of serum, which is required for silica-stimulated cytokine release, neither form of silica caused noticeable elevation of intracellular calcium. Silica, however, did increase the extent of tyrosine phosphorylation, most notably of proteins at approximately 46 and 50 kDa, suggesting activation of a tyrosine kinase pathway. Preincubation of alveolar macrophages for 24 hr with silica-primed human alveolar macrophages for enhanced interleukin-1 beta (IL-1 beta) release stimulated by endotoxin (LPS) that was dose dependent. The enhanced LPS-stimulated release of IL-1 beta correlated with enhanced mitogen-activated protein kinase activity. Taken together, these results indicate that a tyrosine kinase pathway is activated during silica stimulation of human alveolar macrophages.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brown G. P., Monick M., Hunninghake G. W. Fibroblast proliferation induced by silica-exposed human alveolar macrophages. Am Rev Respir Dis. 1988 Jul;138(1):85–89. doi: 10.1164/ajrccm/138.1.85. [DOI] [PubMed] [Google Scholar]
- Chen J., Armstrong L. C., Liu S. J., Gerriets J. E., Last J. A. Silica increases cytosolic free calcium ion concentration of alveolar macrophages in vitro. Toxicol Appl Pharmacol. 1991 Nov;111(2):211–220. doi: 10.1016/0041-008x(91)90025-a. [DOI] [PubMed] [Google Scholar]
- Clark-Lewis I., Sanghera J. S., Pelech S. L. Definition of a consensus sequence for peptide substrate recognition by p44mpk, the meiosis-activated myelin basic protein kinase. J Biol Chem. 1991 Aug 15;266(23):15180–15184. [PubMed] [Google Scholar]
- Dauber J. H., Rossman M. D., Daniele R. P. Bronchoalveolar cell populations in acute sarcoidosis. Observations in smoking and nonsmoking patients. J Lab Clin Med. 1979 Dec;94(6):862–871. [PubMed] [Google Scholar]
- Davis G. S. Pathogenesis of silicosis: current concepts and hypotheses. Lung. 1986;164(3):139–154. doi: 10.1007/BF02713638. [DOI] [PubMed] [Google Scholar]
- Driscoll K. E., Lindenschmidt R. C., Maurer J. K., Higgins J. M., Ridder G. Pulmonary response to silica or titanium dioxide: inflammatory cells, alveolar macrophage-derived cytokines, and histopathology. Am J Respir Cell Mol Biol. 1990 Apr;2(4):381–390. doi: 10.1165/ajrcmb/2.4.381. [DOI] [PubMed] [Google Scholar]
- Fairhurst R. M., Daeipour M., Amaral M. C., Nel A. E. Activation of mitogen-activated protein kinase/ERK-2 in phytohaemagglutin in blasts by recombinant interleukin-2: contrasting features with CD3 activation. Immunology. 1993 May;79(1):112–118. [PMC free article] [PubMed] [Google Scholar]
- Gold M. R., Sanghera J. S., Stewart J., Pelech S. L. Selective activation of p42 mitogen-activated protein (MAP) kinase in murine B lymphoma cell lines by membrane immunoglobulin cross-linking. Evidence for protein kinase C-independent and -dependent mechanisms of activation. Biochem J. 1992 Oct 1;287(Pt 1):269–276. doi: 10.1042/bj2870269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalla B., Hamilton R. F., Scheule R. K., Holian A. Role of extracellular calcium in chrysotile asbestos stimulation of alveolar macrophages. Toxicol Appl Pharmacol. 1990 Jun 1;104(1):130–138. doi: 10.1016/0041-008x(90)90288-6. [DOI] [PubMed] [Google Scholar]
- Kampschmidt R. F., Worthington M. L., 3rd, Mesecher M. I. Release of interleukin-1 (IL-1) and IL-1-like factors from rabbit macrophages with silica. J Leukoc Biol. 1986 Feb;39(2):123–132. doi: 10.1002/jlb.39.2.123. [DOI] [PubMed] [Google Scholar]
- Kelley J. Cytokines of the lung. Am Rev Respir Dis. 1990 Mar;141(3):765–788. doi: 10.1164/ajrccm/141.3.765. [DOI] [PubMed] [Google Scholar]
- Perkins R. C., Scheule R. K., Hamilton R., Gomes G., Freidman G., Holian A. Human alveolar macrophage cytokine release in response to in vitro and in vivo asbestos exposure. Exp Lung Res. 1993 Jan-Mar;19(1):55–65. doi: 10.3109/01902149309071080. [DOI] [PubMed] [Google Scholar]
- Perkins R. C., Scheule R. K., Holian A. In vitro bioactivity of asbestos for the human alveolar macrophage and its modification by IgG. Am J Respir Cell Mol Biol. 1991 Jun;4(6):532–537. doi: 10.1165/ajrcmb/4.6.532. [DOI] [PubMed] [Google Scholar]
- Rojanasakul Y., Wang L., Malanga C. J., Ma J. Y., Banks D. E., Ma J. K. Altered calcium homeostasis and cell injury in silica-exposed alveolar macrophages. J Cell Physiol. 1993 Feb;154(2):310–316. doi: 10.1002/jcp.1041540214. [DOI] [PubMed] [Google Scholar]
- Rom W. N., Bitterman P. B., Rennard S. I., Cantin A., Crystal R. G. Characterization of the lower respiratory tract inflammation of nonsmoking individuals with interstitial lung disease associated with chronic inhalation of inorganic dusts. Am Rev Respir Dis. 1987 Dec;136(6):1429–1434. doi: 10.1164/ajrccm/136.6.1429. [DOI] [PubMed] [Google Scholar]
- Rom W. N., Travis W. D., Brody A. R. Cellular and molecular basis of the asbestos-related diseases. Am Rev Respir Dis. 1991 Feb;143(2):408–422. doi: 10.1164/ajrccm/143.2.408. [DOI] [PubMed] [Google Scholar]
- Roney P. L., Holian A. Possible mechanism of chrysotile asbestos-stimulated superoxide anion production in guinea pig alveolar macrophages. Toxicol Appl Pharmacol. 1989 Aug;100(1):132–144. doi: 10.1016/0041-008x(89)90097-5. [DOI] [PubMed] [Google Scholar]
- Scheule R. K., Holian A. IgG specifically enhances chrysotile asbestos-stimulated superoxide anion production by the alveolar macrophage. Am J Respir Cell Mol Biol. 1989 Oct;1(4):313–318. doi: 10.1165/ajrcmb/1.4.313. [DOI] [PubMed] [Google Scholar]
- Schmidt J. A., Oliver C. N., Lepe-Zuniga J. L., Green I., Gery I. Silica-stimulated monocytes release fibroblast proliferation factors identical to interleukin 1. A potential role for interleukin 1 in the pathogenesis of silicosis. J Clin Invest. 1984 May;73(5):1462–1472. doi: 10.1172/JCI111350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- deShazo R. D. Current concepts about the pathogenesis of silicosis and asbestosis. J Allergy Clin Immunol. 1982 Jul;70(1):41–49. doi: 10.1016/0091-6749(82)90200-7. [DOI] [PubMed] [Google Scholar]





