Abstract
Arylamines, nitroarenes, and azo dyes yield a common type of metabolite, the nitroarene, which produces a hydrolyzable adduct with protein and is closely related to the critical, ultimate toxic and genotoxic metabolite. The target dose as measured by hemoglobin adducts in erythrocytes reflects not only the actual uptake from the environment but also an individual's capacity for metabolic activation and is therefore an improved dosimeter for human exposure. The usefulness of hemoglobin adducts in molecular epidemiology is now widely recognized. With regard to risk assessment, many questions need to be answered. The described experiments in rats address some of these questions. The relationship between binding to hemoglobin in erythrocytes and to proteins in plasma has been found to vary considerably for a number of diamines. The fraction of hydrolyzable adducts out of the total protein adducts formed also varies in both compartments. This indicates that the kind of circulating metabolites and their availability in different compartments is compound specific. This has to do with the complex pattern of competing metabolic pathways, and the role of N-acetylation and deacetylation is emphasized. An example of nonlinear dose dependence adds to the complexity. Analysis of hemoglobin adducts reveals interesting insights into prevailing pathways, which not only apply to the chemical, but may also be useful to assess an individual's metabolic properties. In addition, it is demonstrated that the greater part of erythrocytes and benzidine-hemoglobin adducts are eliminated randomly in rats, i.e., following first-order kinetics.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akahane K., Furuhama K., Inage F., Onodera T. Effects of malotilate on rat erythrocytes. Jpn J Pharmacol. 1987 Sep;45(1):15–25. doi: 10.1254/jjp.45.15. [DOI] [PubMed] [Google Scholar]
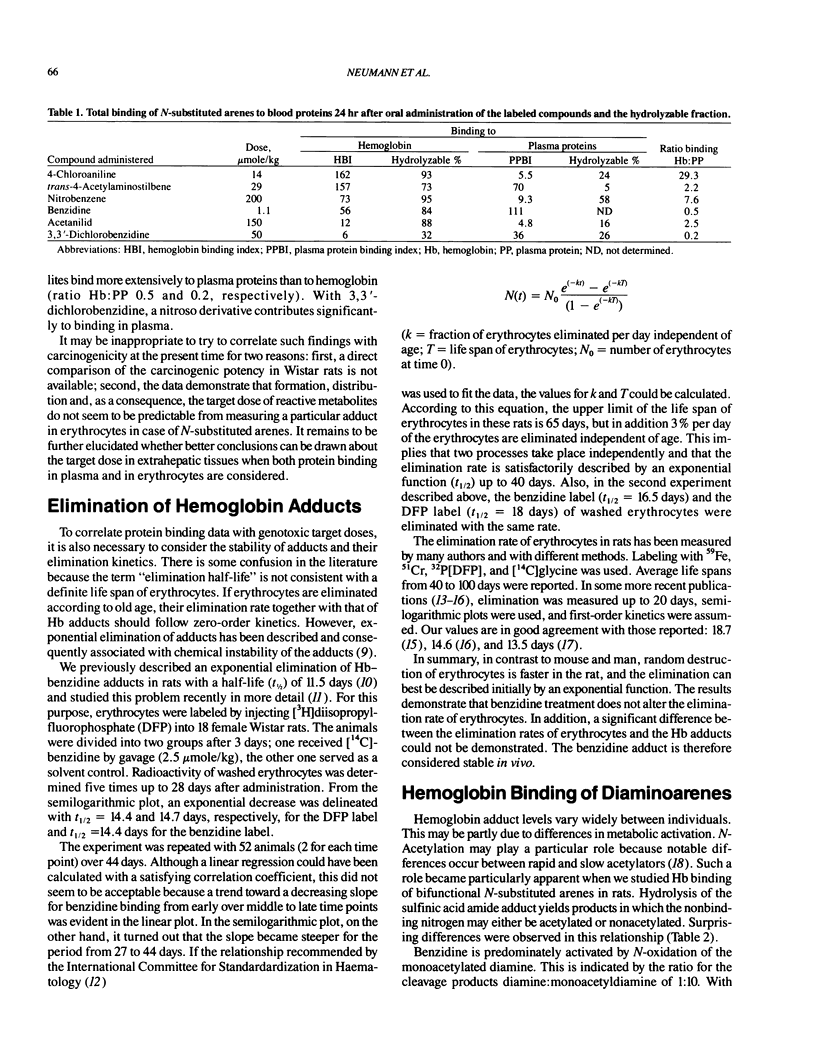
- Bailey E., Brooks A. G., Bird I., Farmer P. B., Street B. Monitoring exposure to 4,4'-methylenedianiline by the gas chromatography-mass spectrometry determination of adducts to hemoglobin. Anal Biochem. 1990 Nov 1;190(2):175–181. doi: 10.1016/0003-2697(90)90177-b. [DOI] [PubMed] [Google Scholar]
- Birner G., Albrecht W., Neumann H. G. Biomonitoring of aromatic amines. III: Hemoglobin binding of benzidine and some benzidine congeners. Arch Toxicol. 1990;64(2):97–102. doi: 10.1007/BF01974393. [DOI] [PubMed] [Google Scholar]
- Birner G., Neumann H. G. Biomonitoring of aromatic amines II: Hemoglobin binding of some monocyclic aromatic amines. Arch Toxicol. 1988;62(2-3):110–115. doi: 10.1007/BF00570128. [DOI] [PubMed] [Google Scholar]
- Cunningham M. L., Burka L. T., Matthews H. B. Metabolism, disposition, and mutagenicity of 2,6-diaminotoluene, a mutagenic noncarcinogen. Drug Metab Dispos. 1989 Nov-Dec;17(6):612–617. [PubMed] [Google Scholar]
- Dölle B., Töpner W., Neumann H. G. Reaction of arylnitroso compounds with mercaptans. Xenobiotica. 1980 Jul-Aug;10(7-8):527–536. doi: 10.3109/00498258009033787. [DOI] [PubMed] [Google Scholar]
- Grantham P. H., Mohan L., Benjamin T., Roller P. P., Miller J. R., Weisburger E. K. Comparison of the metabolism of 2,4-toluenediamine in rats and mice. J Environ Pathol Toxicol. 1979 Dec;3(1-2):149–166. [PubMed] [Google Scholar]
- HJORT P. F., PAPUTCHIS H., CHENEY B. Labeling of red blood cells with radioactive diisopropylfluorophosphate (DFP32): evidence for an initial release of label. J Lab Clin Med. 1960 Mar;55:416–424. [PubMed] [Google Scholar]
- Kennelly J. C., Beland F. A., Kadlubar F. F., Martin C. N. Binding of N-acetylbenzidine and N,N'-diacetylbenzidine to hepatic DNA of rat and hamster in vivo and in vitro. Carcinogenesis. 1984 Mar;5(3):407–412. doi: 10.1093/carcin/5.3.407. [DOI] [PubMed] [Google Scholar]
- Leonard T. B., Graichen M. E., Popp J. A. Dinitrotoluene isomer-specific hepatocarcinogenesis in F344 rats. J Natl Cancer Inst. 1987 Dec;79(6):1313–1319. [PubMed] [Google Scholar]
- Lewalter J., Korallus U. Blood protein conjugates and acetylation of aromatic amines. New findings on biological monitoring. Int Arch Occup Environ Health. 1985;56(3):179–196. doi: 10.1007/BF00396596. [DOI] [PubMed] [Google Scholar]
- Messerly E. A., Fekete J. E., Wade D. R., Sinsheimer J. E. Structure-mutagenicity relationships of benzidine analogues. Environ Mol Mutagen. 1987;10(3):263–274. doi: 10.1002/em.2850100305. [DOI] [PubMed] [Google Scholar]
- Mori M. A., Miyahara T., Taniguchi K., Hasegawa K., Kozuka H., Miyagoshi M., Nagayama T. Mutagenicity of 2,4-dinitrotoluene and its metabolites in Salmonella typhimurium. Toxicol Lett. 1982 Sep;13(1-2):1–5. doi: 10.1016/0378-4274(82)90130-8. [DOI] [PubMed] [Google Scholar]
- Noble N. A., Rothstein G. The Dpg gene: an intracorpuscular modifier of red cell metabolism. Blood. 1986 May;67(5):1210–1214. [PubMed] [Google Scholar]
- Parodi S., Taningher M., Russo P., Pala M., Tamaro M., Monti-Bragadin C. DNA-damaging activity in vivo and bacterial mutagenicity of sixteen aromatic amines and azo-derivatives, as related quantitatively to their carcinogenicity. Carcinogenesis. 1981;2(12):1317–1326. doi: 10.1093/carcin/2.12.1317. [DOI] [PubMed] [Google Scholar]
- Rickert D. E., Long R. M. Metabolism and excretion of 2,4-dinitrotoluene in male and female Fischer 344 rats after different doses. Drug Metab Dispos. 1981 May-Jun;9(3):226–232. [PubMed] [Google Scholar]
- Sabbioni G., Neumann H. G. Biomonitoring of arylamines: hemoglobin adducts of urea and carbamate pesticides. Carcinogenesis. 1990 Jan;11(1):111–115. doi: 10.1093/carcin/11.1.111. [DOI] [PubMed] [Google Scholar]
- Sabbioni G., Neumann H. G. Quantification of haemoglobin binding of 4,4'-methylenebis(2-chloroaniline) (MOCA) in rats. Arch Toxicol. 1990;64(6):451–458. doi: 10.1007/BF01977626. [DOI] [PubMed] [Google Scholar]
- Skipper P. L., Tannenbaum S. R. Protein adducts in the molecular dosimetry of chemical carcinogens. Carcinogenesis. 1990 Apr;11(4):507–518. doi: 10.1093/carcin/11.4.507. [DOI] [PubMed] [Google Scholar]
- Spanggord R. J., Mortelmans K. E., Griffin A. F., Simmon V. F. Mutagenicity in Salmonella typhimurium and structure-activity relationships of wastewater components emanating from the manufacture of trinitrotoluene. Environ Mutagen. 1982;4(2):163–179. doi: 10.1002/em.2860040207. [DOI] [PubMed] [Google Scholar]
- WOHL H., MERSKEY C. ANEMIA IN RATS ON ATHEROGENIC DIETS. Am J Physiol. 1964 Apr;206:765–768. doi: 10.1152/ajplegacy.1964.206.4.765. [DOI] [PubMed] [Google Scholar]
- Wieland E., Neumann H. G. Methemoglobin formation and binding to blood constituents as indicators for the formation, availability and reactivity of activated metabolites derived from trans-4-aminostilbene and related aromatic amines. Arch Toxicol. 1978 Feb 21;40(1):17–35. doi: 10.1007/BF00353276. [DOI] [PubMed] [Google Scholar]
- Wise R. W., Zenser T. V., Kadlubar F. F., Davis B. B. Metabolic activation of carcinogenic aromatic amines by dog bladder and kidney prostaglandin H synthase. Cancer Res. 1984 May;44(5):1893–1897. [PubMed] [Google Scholar]
- Yamazoe Y., Zenser T. V., Miller D. W., Kadlubar F. F. Mechanism of formation and structural characterization of DNA adducts derived from peroxidative activation of benzidine. Carcinogenesis. 1988 Sep;9(9):1635–1641. doi: 10.1093/carcin/9.9.1635. [DOI] [PubMed] [Google Scholar]


