Abstract
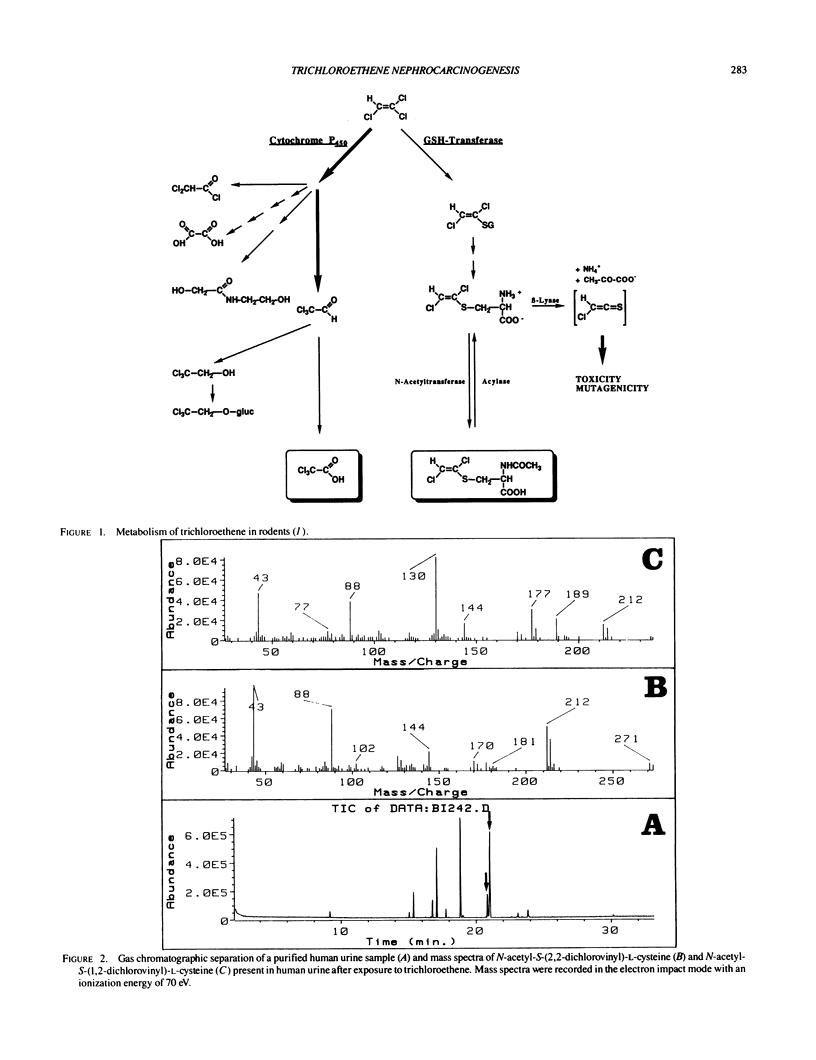
Excretion of mercapturic acids in the urine is indicative of the formation of electrophiles in the metabolism of xenobiotics. The determination of these mercapturic acids thus may be a useful method to estimate the exposure. We identified the nephrotoxic and mutagenic mercapturic acids N-acetyl-S-(1,2-dichlorovinyl)-L- cysteine and N-acetyl-S-(2,2-dichlorovinyl)-L-cysteine in the urine of workers exposed to 1,1,2-trichloroethene. A method to quantify these mercapturic acids by gas chromatography-mass spectrometry-selected ion monitoring was developed and appreciable amounts (2.8-3.8 mumole/L were found in human urine samples. Because deacetylation determines notably the amount of the excreted mercapturic acids, the formation of the resulting cysteine S-conjugates was comparably measured in subcellular fractions of rodent and human kidneys; significant species differences in acylase activity were found. The formation of mutagenic and nephrotoxic metabolites during 1,1,2-trichloroethene metabolism mandates a revision of the risk assessment of trichloroethene exposure.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Commandeur J. N., Vermeulen N. P. Identification of N-acetyl(2,2-dichlorovinyl)- and N-acetyl(1,2-dichlorovinyl)-L-cysteine as two regioisomeric mercapturic acids of trichloroethylene in the rat. Chem Res Toxicol. 1990 May-Jun;3(3):212–218. doi: 10.1021/tx00015a005. [DOI] [PubMed] [Google Scholar]
- Dekant W., Koob M., Henschler D. Metabolism of trichloroethene--in vivo and in vitro evidence for activation by glutathione conjugation. Chem Biol Interact. 1990;73(1):89–101. doi: 10.1016/0009-2797(90)90110-9. [DOI] [PubMed] [Google Scholar]
- Dekant W. Metabolic conversion of tri- and tetrachloroethylene: formation and deactivation of genotoxic intermediates. Dev Toxicol Environ Sci. 1986;12:211–221. [PubMed] [Google Scholar]
- Dekant W., Vamvakas S., Berthold K., Schmidt S., Wild D., Henschler D. Bacterial beta-lyase mediated cleavage and mutagenicity of cysteine conjugates derived from the nephrocarcinogenic alkenes trichloroethylene, tetrachloroethylene and hexachlorobutadiene. Chem Biol Interact. 1986 Oct 15;60(1):31–45. doi: 10.1016/0009-2797(86)90015-3. [DOI] [PubMed] [Google Scholar]
- Elcombe C. R., Rose M. S., Pratt I. S. Biochemical, histological, and ultrastructural changes in rat and mouse liver following the administration of trichloroethylene: possible relevance to species differences in hepatocarcinogenicity. Toxicol Appl Pharmacol. 1985 Jul;79(3):365–376. doi: 10.1016/0041-008x(85)90135-8. [DOI] [PubMed] [Google Scholar]
- Koob M., Dekant W. Bioactivation of xenobiotics by formation of toxic glutathione conjugates. Chem Biol Interact. 1991;77(2):107–136. doi: 10.1016/0009-2797(91)90068-i. [DOI] [PubMed] [Google Scholar]


