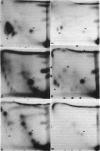
Abstract
White blood cell DNA adducts were measured in coke oven workers, in residents from the area next to the coke oven in Silesia, Poland (highly industrialized region), and in residents from the rural area of Poland using the 32P-postlabeling technique. This method detected aromatic adducts including adducts formed by polycyclic aromatic hydrocarbons (PAHs). Highest levels of adducts in DNA were seen in the group of coke battery workers (6.9 adducts/10(8) nucleotides). Seasonal variations in levels of DNA adducts were observed both in residents of the district near the coke oven area and individuals from the rural area of Poland. Blood samples collected from people living near the coke oven in winter showed much higher levels of DNA adducts than blood samples obtained in summer (5.0 adducts/10(8) nucleotides in winter and 1.4 adducts/10(8) nucleotides in summer). The difference in the level of DNA adducts between winter and summer was smaller in the group of people living in the rural area (3.2 adducts/10(8) and 2.2 adducts/10(8), respectively). In most cases the levels of X-spots correlated with the levels of other DNA adducts. Correlation coefficients(r) between the levels of X-spots and other adducts ranged between 0.46 and 0.74 (p < 0.05), except for coke oven workers where no correlation was observed.
Full text
PDF
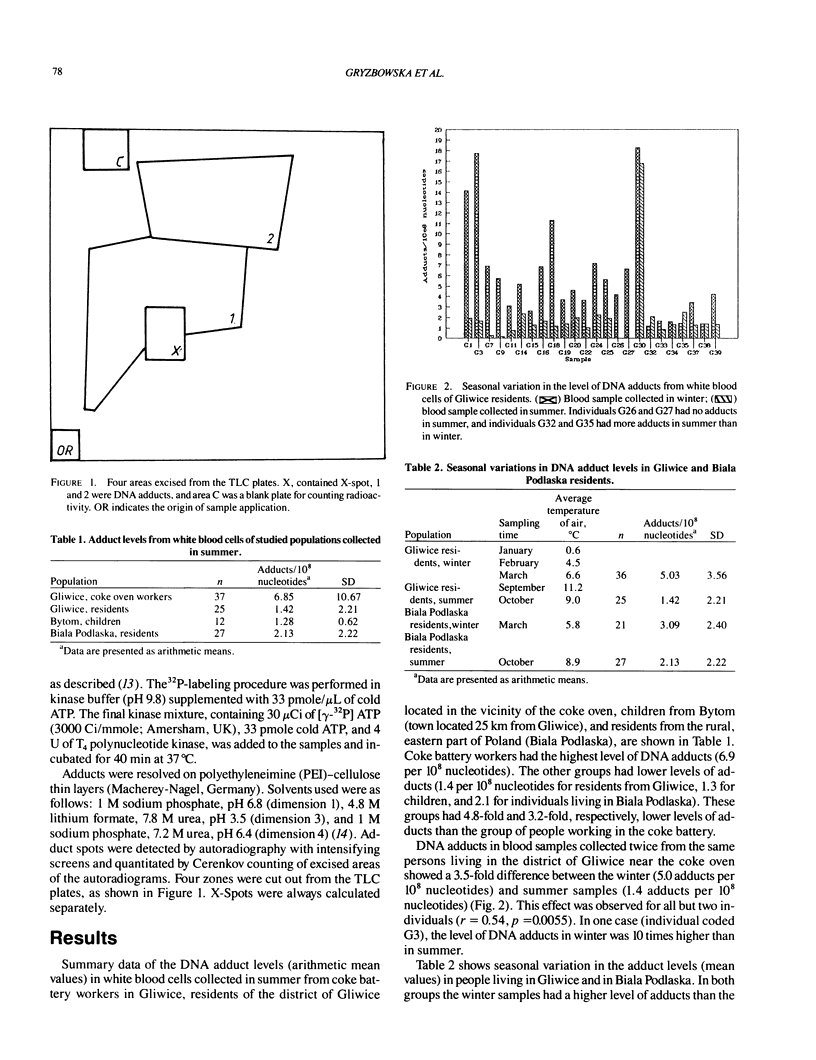
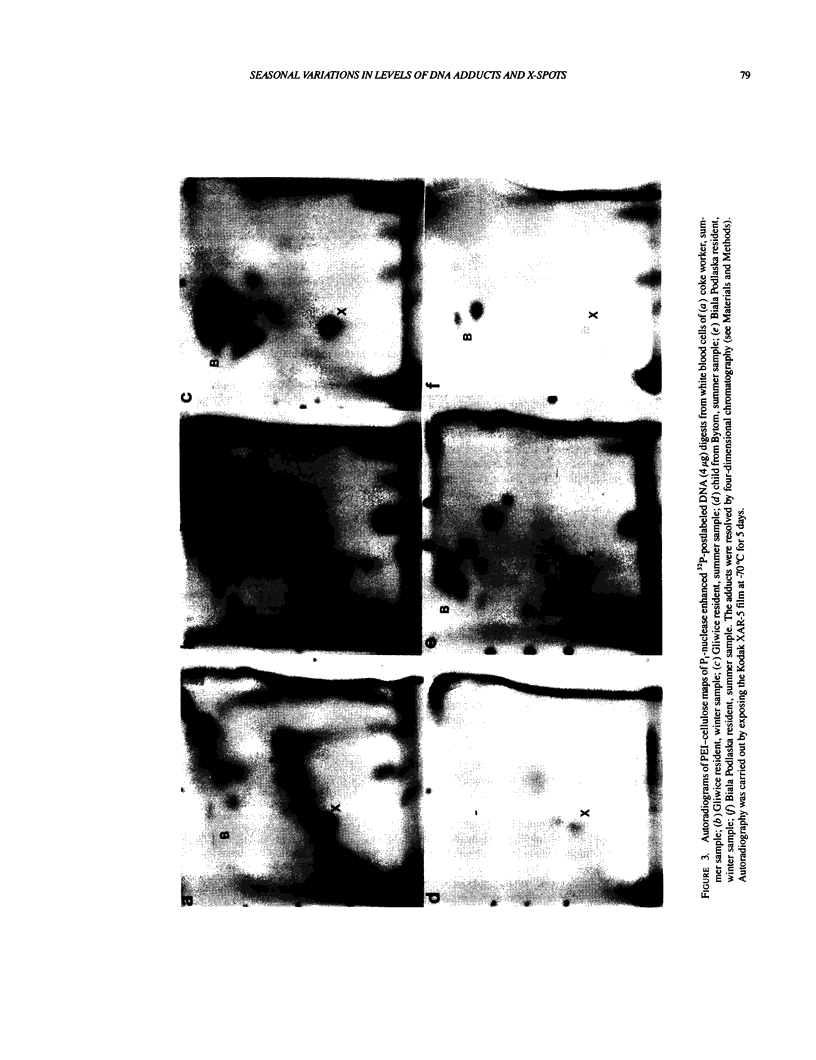


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Autrup H. Carcinogen metabolism in cultured human tissues and cells. Carcinogenesis. 1990 May;11(5):707–712. doi: 10.1093/carcin/11.5.707. [DOI] [PubMed] [Google Scholar]
- Everson R. B., Randerath E., Santella R. M., Cefalo R. C., Avitts T. A., Randerath K. Detection of smoking-related covalent DNA adducts in human placenta. Science. 1986 Jan 3;231(4733):54–57. doi: 10.1126/science.3941892. [DOI] [PubMed] [Google Scholar]
- Faletto M. B., Maccubbin A. E., Koser P. L., Vangalio J. A., Gurtoo H. L. Induction of aryl hydrocarbon hydroxylase and DNA adduct formation in parental and carcinogen transformed C3H/10T1/2 clone 8 cells by benzo[a]pyrene. Cancer Biochem Biophys. 1989 May;10(3):197–205. [PubMed] [Google Scholar]
- Fukino H., Mimura S., Inoue K., Yamane Y. Mutagenicity of airborne particles. Mutat Res. 1982 Oct-Nov;102(3):237–247. doi: 10.1016/0165-1218(82)90133-1. [DOI] [PubMed] [Google Scholar]
- Gupta R. C., Reddy M. V., Randerath K. 32P-postlabeling analysis of non-radioactive aromatic carcinogen--DNA adducts. Carcinogenesis. 1982;3(9):1081–1092. doi: 10.1093/carcin/3.9.1081. [DOI] [PubMed] [Google Scholar]
- Harris C. C., Vahakangas K., Newman M. J., Trivers G. E., Shamsuddin A., Sinopoli N., Mann D. L., Wright W. E. Detection of benzo[a]pyrene diol epoxide-DNA adducts in peripheral blood lymphocytes and antibodies to the adducts in serum from coke oven workers. Proc Natl Acad Sci U S A. 1985 Oct;82(19):6672–6676. doi: 10.1073/pnas.82.19.6672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haugen A., Becher G., Benestad C., Vahakangas K., Trivers G. E., Newman M. J., Harris C. C. Determination of polycyclic aromatic hydrocarbons in the urine, benzo(a)pyrene diol epoxide-DNA adducts in lymphocyte DNA, and antibodies to the adducts in sera from coke oven workers exposed to measured amounts of polycyclic aromatic hydrocarbons in the work atmosphere. Cancer Res. 1986 Aug;46(8):4178–4183. [PubMed] [Google Scholar]
- Hemminki K., Grzybowska E., Chorazy M., Twardowska-Saucha K., Sroczynski J. W., Putman K. L., Randerath K., Phillips D. H., Hewer A. Aromatic DNA adducts in white blood cells of coke workers. Int Arch Occup Environ Health. 1990;62(6):467–470. doi: 10.1007/BF00379065. [DOI] [PubMed] [Google Scholar]
- Hemminki K., Grzybowska E., Chorazy M., Twardowska-Saucha K., Sroczynski J. W., Putman K. L., Randerath K., Phillips D. H., Hewer A., Santella R. M. DNA adducts in human environmentally exposed to aromatic compounds in an industrial area of Poland. Carcinogenesis. 1990 Jul;11(7):1229–1231. doi: 10.1093/carcin/11.7.1229. [DOI] [PubMed] [Google Scholar]
- Hemminki K., Randerath K., Reddy M. V., Putman K. L., Santella R. M., Perera F. P., Young T. L., Phillips D. H., Hewer A., Savela K. Postlabeling and immunoassay analysis of polycyclic aromatic hydrocarbons--adducts of deoxyribonucleic acid in white blood cells of foundry workers. Scand J Work Environ Health. 1990 Jun;16(3):158–162. doi: 10.5271/sjweh.1798. [DOI] [PubMed] [Google Scholar]
- Hincal F. Effects of exposure to air pollution and smoking on the placental aryl hydrocarbon hydroxylase (AHH) activity. Arch Environ Health. 1986 Nov-Dec;41(6):377–383. doi: 10.1080/00039896.1986.9935782. [DOI] [PubMed] [Google Scholar]
- Motykiewicz G., Szeliga J., Cimander B., Choraźy M. Seasonal variations in mutagenic activity of air pollutants at an industrial district of Silesia. Mutat Res. 1989 Jun;223(2):243–251. doi: 10.1016/0165-1218(89)90052-9. [DOI] [PubMed] [Google Scholar]
- Perera F. P., Hemminki K., Young T. L., Brenner D., Kelly G., Santella R. M. Detection of polycyclic aromatic hydrocarbon-DNA adducts in white blood cells of foundry workers. Cancer Res. 1988 Apr 15;48(8):2288–2291. [PubMed] [Google Scholar]
- Perera F. P., Jeffrey A. M., Brandt-Rauf P. W., Brenner D., Mayer J. L., Smith S. J., Latriano L., Hemminki K., Santella R. M. Molecular epidemiology and cancer prevention. Cancer Detect Prev. 1990;14(6):639–645. [PubMed] [Google Scholar]
- Perera F. P. The significance of DNA and protein adducts in human biomonitoring studies. Mutat Res. 1988 May-Aug;205(1-4):255–269. doi: 10.1016/0165-1218(88)90021-3. [DOI] [PubMed] [Google Scholar]
- Phillips D. H., Hewer A., Grover P. L. Aromatic DNA adducts in human bone marrow and peripheral blood leukocytes. Carcinogenesis. 1986 Dec;7(12):2071–2075. doi: 10.1093/carcin/7.12.2071. [DOI] [PubMed] [Google Scholar]
- Phillips D. H., Hewer A., Martin C. N., Garner R. C., King M. M. Correlation of DNA adduct levels in human lung with cigarette smoking. Nature. 1988 Dec 22;336(6201):790–792. doi: 10.1038/336790a0. [DOI] [PubMed] [Google Scholar]
- Reddy M. V., Randerath K. Nuclease P1-mediated enhancement of sensitivity of 32P-postlabeling test for structurally diverse DNA adducts. Carcinogenesis. 1986 Sep;7(9):1543–1551. doi: 10.1093/carcin/7.9.1543. [DOI] [PubMed] [Google Scholar]
- Weinstein I. B. The origins of human cancer: molecular mechanisms of carcinogenesis and their implications for cancer prevention and treatment--twenty-seventh G.H.A. Clowes memorial award lecture. Cancer Res. 1988 Aug 1;48(15):4135–4143. [PubMed] [Google Scholar]
- Wiencke J. K., McDowell M. L., Bodell W. J. Molecular dosimetry of DNA adducts and sister chromatid exchanges in human lymphocytes treated with benzo[a]pyrene. Carcinogenesis. 1990 Sep;11(9):1497–1502. doi: 10.1093/carcin/11.9.1497. [DOI] [PubMed] [Google Scholar]