Abstract
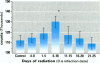
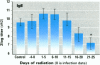
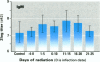
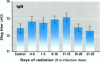
To assess the possibility that increases in UV-B exposure on the earth's surface could lead to impaired resistance to several infectious diseases, we studied the effect of UV-B exposure on resistance against Trichinella spiralis. Wistar rats, orally infected with T. spiralis larvae, were exposed to suberythemal doses of UV-B radiation daily for 5 days at different time periods before or after infection. A significant increase in the number of Trichinella larvae was found in the carcasses of rats irradiated with UV-B between 6 and 10 days after infection. These data indicate that exposure to UV-B radiation suppresses the resistance to a parasitic infection. We suggested that UV-B radiation especially suppresses cellular immune responses against these worms because specific IgM, IgG, and IgE titers were not significantly altered by UV-B exposure. These data indicate that UV-B irradiation plays a role in the course of infection with T. spiralis, which suggests that increases of UV-B exposure might also lead to problems with other infectious diseases and might affect vaccination because of the interaction of UV-B irradiation with memory T-cells.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cox F. E., Liew F. Y. T-cell subsets and cytokines in parasitic infections. Immunol Today. 1992 Nov;13(11):445–448. doi: 10.1016/0167-5699(92)90072-F. [DOI] [PubMed] [Google Scholar]
- Daynes R. A., Spellman C. W. Evidence for the generation of suppressor cells by ultraviolet radiation. Cell Immunol. 1977 Jun 1;31(1):182–187. doi: 10.1016/0008-8749(77)90018-1. [DOI] [PubMed] [Google Scholar]
- De Fabo E. C., Noonan F. P. Mechanism of immune suppression by ultraviolet irradiation in vivo. I. Evidence for the existence of a unique photoreceptor in skin and its role in photoimmunology. J Exp Med. 1983 Jul 1;158(1):84–98. doi: 10.1084/jem.158.1.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denkins Y., Fidler I. J., Kripke M. L. Exposure of mice to UV-B radiation suppresses delayed hypersensitivity to Candida albicans. Photochem Photobiol. 1989 May;49(5):615–619. doi: 10.1111/j.1751-1097.1989.tb08432.x. [DOI] [PubMed] [Google Scholar]
- Fears T. R., Scotto J. Estimating increases in skin cancer morbidity due to increases in ultraviolet radiation exposure. Cancer Invest. 1983;1(2):119–126. doi: 10.3109/07357908309042414. [DOI] [PubMed] [Google Scholar]
- Giannini M. S. Suppression of pathogenesis in cutaneous leishmaniasis by UV irradiation. Infect Immun. 1986 Mar;51(3):838–843. doi: 10.1128/iai.51.3.838-843.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goettsch W., Garssen J., de Gruijl F. R., van Loveren H. UV-B and the immune system. A review with special emphasis on T cell-mediated immunity. Thymus. 1993 Mar;21(2):93–114. [PubMed] [Google Scholar]
- Grover D., Zigman S. Coloration of human lenses by near ultraviolet photo-oxidized tryptophan. Exp Eye Res. 1972 Jan;13(1):70–76. doi: 10.1016/0014-4835(72)90126-1. [DOI] [PubMed] [Google Scholar]
- Gurish M. F., Lynch D. H., Daynes R. A. Changes in antigen-presenting cell function in the spleen and lymph nodes of ultraviolet-irradiated mice. Transplantation. 1982 Mar;33(3):280–284. doi: 10.1097/00007890-198203000-00014. [DOI] [PubMed] [Google Scholar]
- Kelfkens G., de Gruijl F. R., van der Leun J. C. Ozone depletion and increase in annual carcinogenic ultraviolet dose. Photochem Photobiol. 1990 Oct;52(4):819–823. doi: 10.1111/j.1751-1097.1990.tb08687.x. [DOI] [PubMed] [Google Scholar]
- Kligman A. M. Perspectives and problems in cutaneous gerontology. J Invest Dermatol. 1979 Jul;73(1):39–46. doi: 10.1111/1523-1747.ep12532758. [DOI] [PubMed] [Google Scholar]
- Kripke M. L., Morison W. L. Studies on the mechanism of systemic suppression of contact hypersensitivity by UVB radiation. II. Differences in the suppression of delayed and contact hypersensitivity in mice. J Invest Dermatol. 1986 May;86(5):543–549. doi: 10.1111/1523-1747.ep12355000. [DOI] [PubMed] [Google Scholar]
- Letvin N. L., Nepom J. T., Greene M. I., Benacerraf B., Germain R. N. Loss of Ia-bearing splenic adherent cells after whole body ultraviolet irradiation. J Immunol. 1980 Dec;125(6):2550–2554. [PubMed] [Google Scholar]
- Maitchouk I. F. Trachoma and cataract: two WHO targets. Int Nurs Rev. 1985 Jan-Feb;32(1):23–25. [PubMed] [Google Scholar]
- Mosmann T. R., Cherwinski H., Bond M. W., Giedlin M. A., Coffman R. L. Two types of murine helper T cell clone. I. Definition according to profiles of lymphokine activities and secreted proteins. J Immunol. 1986 Apr 1;136(7):2348–2357. [PubMed] [Google Scholar]
- Noonan F. P., Bucana C., Sauder D. N., De Fabo E. C. Mechanism of systemic immune suppression by UV irradiation in vivo. II. The UV effects on number and morphology of epidermal Langerhans cells and the UV-induced suppression of contact hypersensitivity have different wavelength dependencies. J Immunol. 1984 May;132(5):2408–2416. [PubMed] [Google Scholar]
- Pitts D. G., Kay K. R. The photo-ophthalmic threshold for the rabbit. Am J Optom Arch Am Acad Optom. 1969 Aug;46(8):561–572. doi: 10.1097/00006324-196908000-00001. [DOI] [PubMed] [Google Scholar]
- Rivas J. M., Ullrich S. E. Systemic suppression of delayed-type hypersensitivity by supernatants from UV-irradiated keratinocytes. An essential role for keratinocyte-derived IL-10. J Immunol. 1992 Dec 15;149(12):3865–3871. [PubMed] [Google Scholar]
- Ruitenberg E. J., Elgersma A. Absence of intestinal mast cell response in congenitally athymic mice during Trichinella spiralis infection. Nature. 1976 Nov 18;264(5583):258–260. doi: 10.1038/264258a0. [DOI] [PubMed] [Google Scholar]
- Simon J. C., Cruz P. D., Jr, Bergstresser P. R., Tigelaar R. E. Low dose ultraviolet B-irradiated Langerhans cells preferentially activate CD4+ cells of the T helper 2 subset. J Immunol. 1990 Oct 1;145(7):2087–2091. [PubMed] [Google Scholar]
- Simon J. C., Tigelaar R. E., Bergstresser P. R., Edelbaum D., Cruz P. D., Jr Ultraviolet B radiation converts Langerhans cells from immunogenic to tolerogenic antigen-presenting cells. Induction of specific clonal anergy in CD4+ T helper 1 cells. J Immunol. 1991 Jan 15;146(2):485–491. [PubMed] [Google Scholar]
- Tokura Y., Miyachi Y., Takigawa M., Yamada M. Ultraviolet-induced suppressor T cells and factor(s) in murine contact photosensitivity. I. Biological and immunochemical characterization of factor(s) extracted from suppressor T cells. Cell Immunol. 1987 Dec;110(2):305–320. doi: 10.1016/0008-8749(87)90125-0. [DOI] [PubMed] [Google Scholar]
- Vos J. G., Ruitenberg E. J., Van Basten N., Buys J., Elgersma A., Kruizinga W. The athymic nude rat. IV. Immunocytochemical study to detect T-cells, and immunological and histopathological reactions against Trichinella spiralis. Parasite Immunol. 1983 Mar;5(2):195–215. doi: 10.1111/j.1365-3024.1983.tb00737.x. [DOI] [PubMed] [Google Scholar]
- Vos J. G., de Klerk A., Krajnc E. I., Kruizinga W., van Ommen B., Rozing J. Toxicity of bis(tri-n-butyltin)oxide in the rat. II. Suppression of thymus-dependent immune responses and of parameters of nonspecific resistance after short-term exposure. Toxicol Appl Pharmacol. 1984 Sep 30;75(3):387–408. doi: 10.1016/0041-008x(84)90177-7. [DOI] [PubMed] [Google Scholar]