Abstract
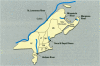
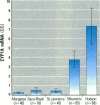
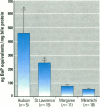
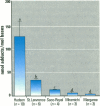
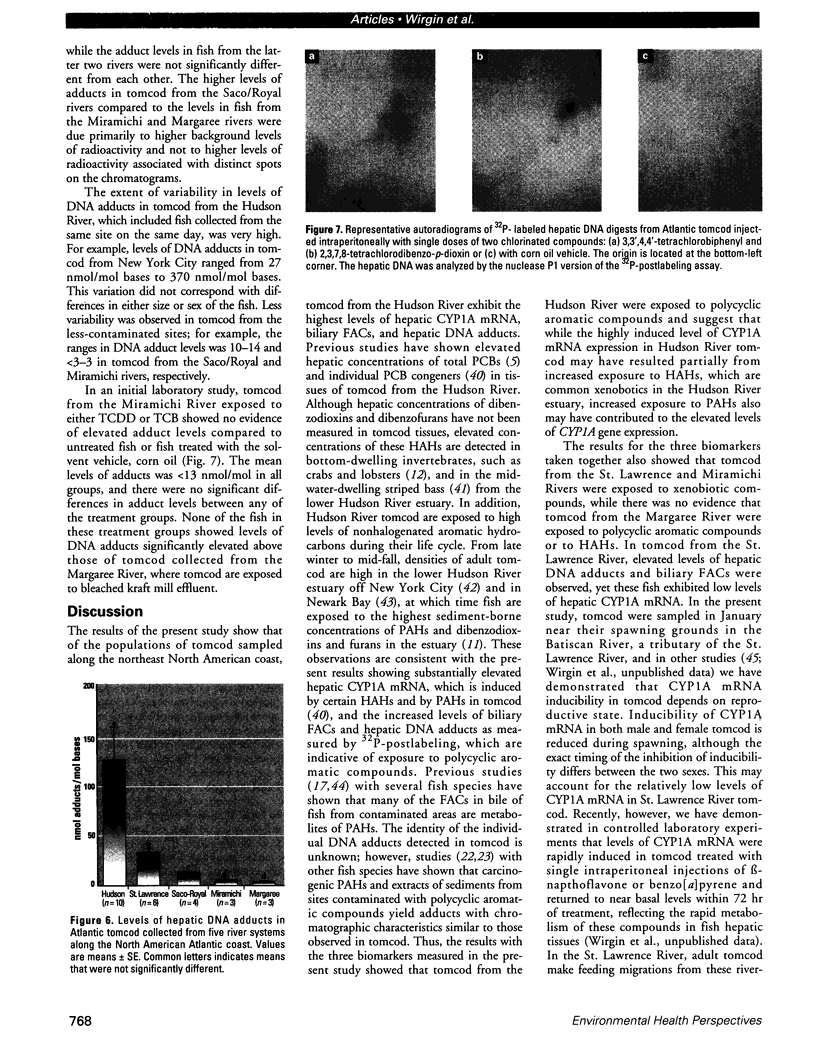
We determined levels of hepatic cytochrome P4501A (CYP1A) mRNA, hepatic DNA adducts, and fluorescent aromatic compounds (FACs) in bile, a measure of exposure to polyaromatic hydrocarbons, in Atlantic tomcod from six river systems ranging from highly polluted to relatively pristine on the northeast North American coast (the Hudson River, New York; the St. Lawrence River, Quebec; the Miramichi River, New Brunswick; the Saco and Royal rivers, Maine; and the Margaree River, Nova Scotia). Hudson River tomcod showed the greatest response for all parameters, and tomcod from the Margaree River exhibited the least response. Tomcod from the Miramichi River exhibited marked induction of CYP1A mRNA but low levels of hepatic DNA adducts and biliary FACs, whereas fish from the St. Lawrence River showed no induction of CYP1A mRNA and moderately elevated levels of DNA adducts and biliary FACs. In tomcod from the Hudson and Miramichi rivers, the levels of CYP1A mRNA were 28 times and 14 times, respectively, as great as the levels in fish from the St. Lawrence, Saco/Royal, and Margaree rivers. Mean levels of DNA adducts varied from 120 nmol adducts/mol bases in Hudson River tomcod to < 3 nmol adducts/mol bases in fish from the Miramichi and Margaree rivers. Concentrations of FACs in the bile of tomcod from the Hudson and St. Lawrence rivers were 8 and 1.8 times, respectively, as great as the concentrations in tomcod from the Miramichi River and Margaree River. In tomcod from the Hudson River, all three biomarkers were markedly elevated; in the St. Lawrence River two biomarkers were elevated, in the Miramichi River one was elevated, but no biomarker was substantially elevated in fish from the Saco/Royal and Margaree rivers. Elevated levels of hepatic DNA adducts and biliary FACs in tomcod from the Hudson River suggest increased exposure to PAHs, consistent with previous studies.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chan Y. L., Gutell R., Noller H. F., Wool I. G. The nucleotide sequence of a rat 18 S ribosomal ribonucleic acid gene and a proposal for the secondary structure of 18 S ribosomal ribonucleic acid. J Biol Chem. 1984 Jan 10;259(1):224–230. [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Collier T. K., Singh S. V., Awasthi Y. C., Varanasi U. Hepatic xenobiotic metabolizing enzymes in two species of benthic fish showing different prevalences of contaminant-associated liver neoplasms. Toxicol Appl Pharmacol. 1992 Apr;113(2):319–324. doi: 10.1016/0041-008x(92)90131-b. [DOI] [PubMed] [Google Scholar]
- Collier T. K., Varanasi U. Hepatic activities of xenobiotic metabolizing enzymes and biliary levels of xenobiotics in English sole (Parophrys vetulus) exposed to environmental contaminants. Arch Environ Contam Toxicol. 1991 May;20(4):462–473. doi: 10.1007/BF01065834. [DOI] [PubMed] [Google Scholar]
- Harshbarger J. C., Clark J. B. Epizootiology of neoplasms in bony fish of North America. Sci Total Environ. 1990 May 1;94(1-2):1–32. doi: 10.1016/0048-9697(90)90362-x. [DOI] [PubMed] [Google Scholar]
- Klauda R. J., Peck T. H., Rice G. K. Accumulation of polychlorinated biphenyls in Atlantic tomcod (Microgadus tomcod) collected from the Hudson River estuary, New York. Bull Environ Contam Toxicol. 1981 Dec;27(6):829–835. doi: 10.1007/BF01611103. [DOI] [PubMed] [Google Scholar]
- Krahn M. M., Burrows D. G., MacLeod W. D., Jr, Malins D. C. Determination of individual metabolites of aromatic compounds in hydrolyzed bile of English sole (Parophrys vetulus) from polluted sites in Puget Sound, Washington. Arch Environ Contam Toxicol. 1987 Sep;16(5):511–522. doi: 10.1007/BF01055807. [DOI] [PubMed] [Google Scholar]
- Krahn M. M., Rhodes L. D., Myers M. S., Moore L. K., MacLeod W. D., Jr, Malins D. C. Associations between metabolites of aromatic compounds in bile and the occurrence of hepatic lesions in English sole (Parophrys vetulus) from Puget Sound, Washington. Arch Environ Contam Toxicol. 1986 Jan;15(1):61–67. doi: 10.1007/BF01055249. [DOI] [PubMed] [Google Scholar]
- Kreamer G. L., Squibb K., Gioeli D., Garte S. J., Wirgin I. Cytochrome P450IA mRNA expression in feral Hudson River tomcod. Environ Res. 1991 Jun;55(1):64–78. doi: 10.1016/s0013-9351(05)80141-0. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Maronpot R. R., Foley J. F., Takahashi K., Goldsworthy T., Clark G., Tritscher A., Portier C., Lucier G. Dose response for TCDD promotion of hepatocarcinogenesis in rats initiated with DEN: histologic, biochemical, and cell proliferation endpoints. Environ Health Perspect. 1993 Dec;101(7):634–642. doi: 10.1289/ehp.93101634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nebert D. W., Petersen D. D., Puga A. Human AH locus polymorphism and cancer: inducibility of CYP1A1 and other genes by combustion products and dioxin. Pharmacogenetics. 1991 Nov;1(2):68–78. doi: 10.1097/00008571-199111000-00003. [DOI] [PubMed] [Google Scholar]
- Reichert W. L., Stein J. E., French B., Goodwin P., Varanasi U. Storage phosphor imaging technique for detection and quantitation of DNA adducts measured by the 32P-postlabeling assay. Carcinogenesis. 1992 Aug;13(8):1475–1479. doi: 10.1093/carcin/13.8.1475. [DOI] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Stein J. E., Reichert W. L., French B., Varanasi U. 32P-postlabeling analysis of DNA adduct formation and persistence in English sole (Pleuronectes vetulus) exposed to benzo[a]pyrene and 7H-dibenzo[c,g]carbazole. Chem Biol Interact. 1993 Jul;88(1):55–69. doi: 10.1016/0009-2797(93)90084-c. [DOI] [PubMed] [Google Scholar]
- Varanasi U., Reichert W. L., Stein J. E. 32P-postlabeling analysis of DNA adducts in liver of wild English sole (Parophrys vetulus) and winter flounder (Pseudopleuronectes americanus). Cancer Res. 1989 Mar 1;49(5):1171–1177. [PubMed] [Google Scholar]
- Varanasi U., Stein J. E. Disposition of xenobiotic chemicals and metabolites in marine organisms. Environ Health Perspect. 1991 Jan;90:93–100. doi: 10.1289/ehp.90-1519508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahl G. M., Stern M., Stark G. R. Efficient transfer of large DNA fragments from agarose gels to diazobenzyloxymethyl-paper and rapid hybridization by using dextran sulfate. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3683–3687. doi: 10.1073/pnas.76.8.3683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wirgin I., Currie D., Garte S. J. Activation of the K-ras oncogene in liver tumors of Hudson River tomcod. Carcinogenesis. 1989 Dec;10(12):2311–2315. doi: 10.1093/carcin/10.12.2311. [DOI] [PubMed] [Google Scholar]