Abstract
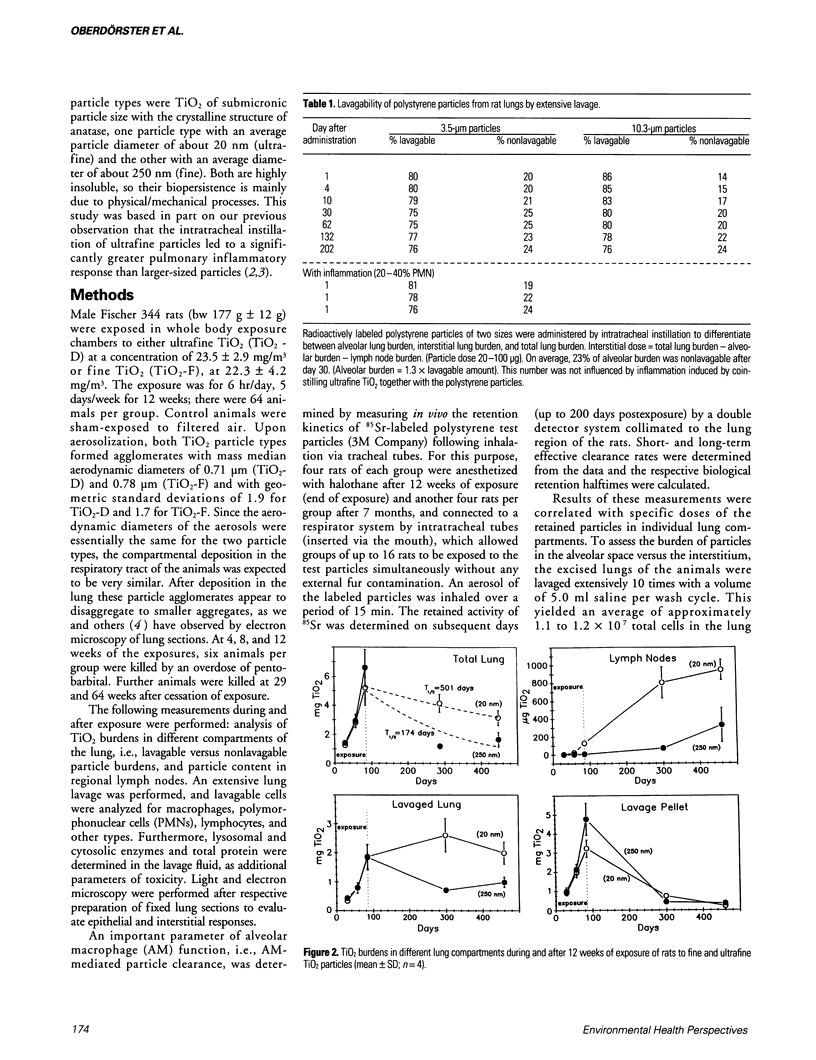
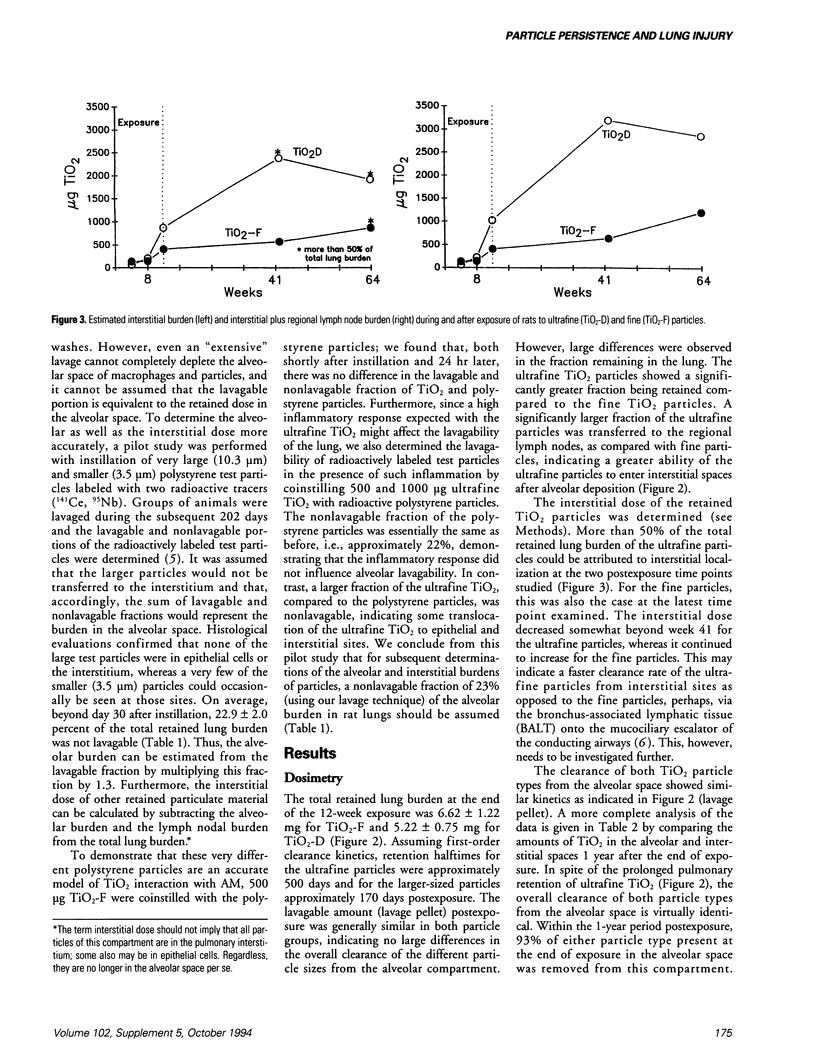
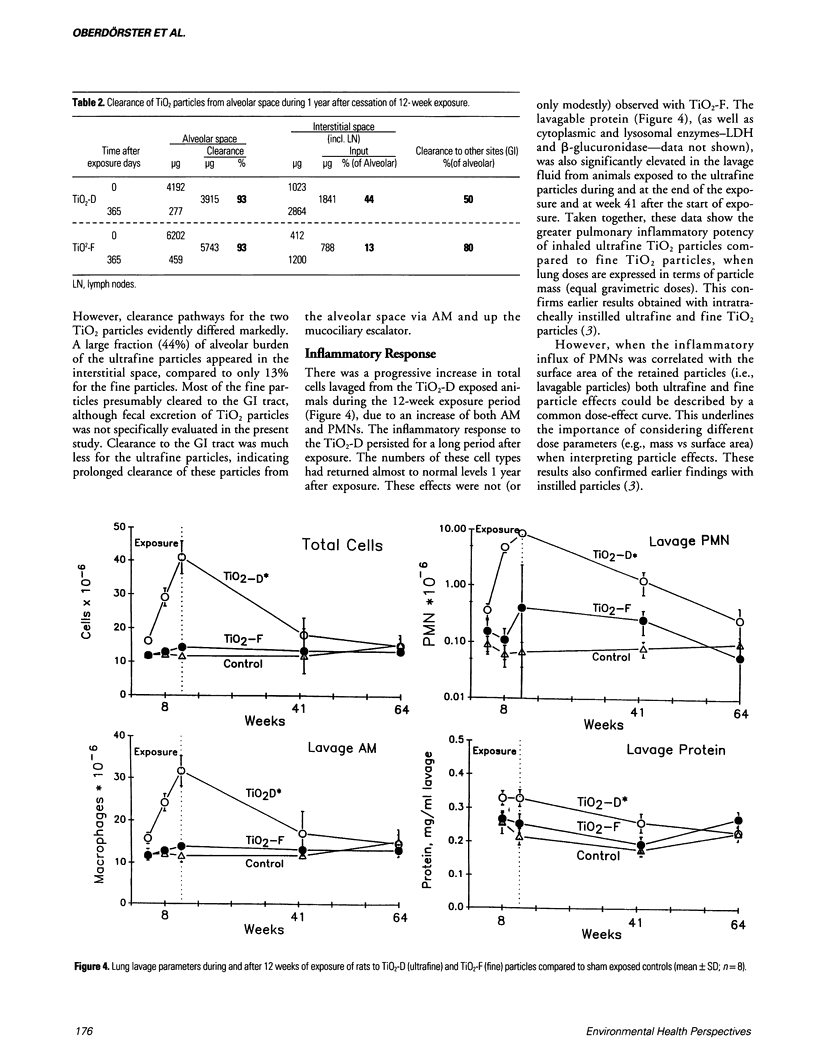
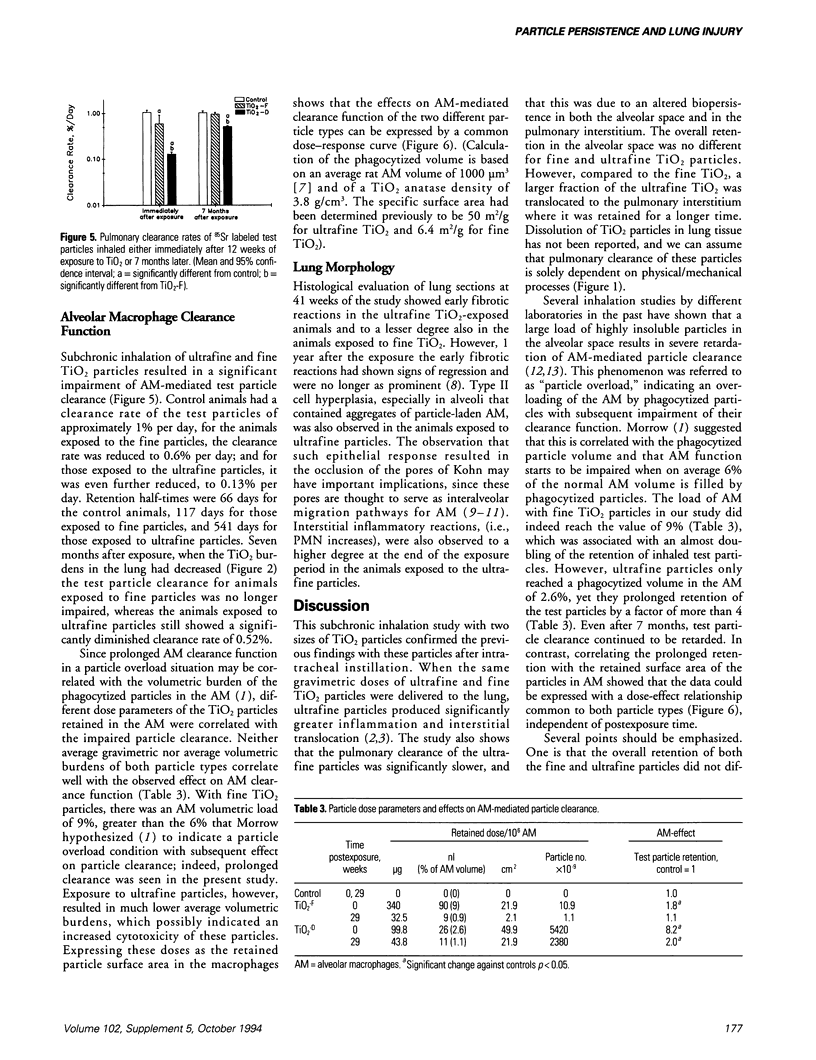
Dosimetry parameters such as deposition, clearance, retention, and translocation and dissolution of inhaled particles in and to different lung compartments may be important for the persistence of particles in the lung and may correlate with adverse pulmonary effects. We investigated such correlations using a model involving TiO2 particles of two particle sizes (20 nm diameter, ultrafine; 250 nm diameter, fine) of the same crystalline structure (anatase). A 12-week inhalation experiment in rats resulted in a similar mass deposition of the two particle types in the lower respiratory tract. The ultrafine particles elicited a persistently high inflammatory reaction in the lungs of the animals compared to the larger-sized particles. In the postexposure period (up to 1 year) retention in the alveolar space per se was not different between fine and ultrafine TiO2. However, the following differences between the particle types were noted: a significantly different total pulmonary retention, both quantitatively (significantly prolonged retention of the ultrafine TiO2) and qualitatively (increased translocation to the pulmonary interstitium and persistence there of the ultrafine TiO2); greater epithelial effects (Type II cell proliferation; occlusion of pores of Kohn) and the beginning of interstitial fibrotic foci with ultrafine TiO2; significant sustained impairment of alveolar macrophage function after ultrafine TiO2 exposure as measured by the clearance of test particles. A correlation between particle surface area and effects was observed. A comparison of the adverse reactions with dosimetric parameters of TiO2 in different lung compartments in the postexposure period showed a correlation of the persistence of effects in both the alveolar and interstitial space with the persistence of particles in the respective compartment.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adamson I. Y., Letourneau H. L., Bowden D. H. Enhanced macrophage-fibroblast interactions in the pulmonary interstitium increases fibrosis after silica injection to monocyte-depleted mice. Am J Pathol. 1989 Feb;134(2):411–418. [PMC free article] [PubMed] [Google Scholar]
- Dethloff L. A., Lehnert B. E. Pulmonary interstitial macrophages: isolation and flow cytometric comparisons with alveolar macrophages and blood monocytes. J Leukoc Biol. 1988 Jan;43(1):80–90. doi: 10.1002/jlb.43.1.80. [DOI] [PubMed] [Google Scholar]
- Driscoll K. E., Maurer J. K. Cytokine and growth factor release by alveolar macrophages: potential biomarkers of pulmonary toxicity. Toxicol Pathol. 1991;19(4 Pt 1):398–405. doi: 10.1177/0192623391019004-108. [DOI] [PubMed] [Google Scholar]
- Ferin J. Pulmonary alveolar pores and alveolar macrophage-mediated particle clearance. Anat Rec. 1982 Jun;203(2):265–272. doi: 10.1002/ar.1092030208. [DOI] [PubMed] [Google Scholar]
- Green G. M. Alveolobronchiolar transport mechanisms. Arch Intern Med. 1973 Jan;131(1):109–114. [PubMed] [Google Scholar]
- Mauderly J. L., Jones R. K., Griffith W. C., Henderson R. F., McClellan R. O. Diesel exhaust is a pulmonary carcinogen in rats exposed chronically by inhalation. Fundam Appl Toxicol. 1987 Aug;9(2):208–221. doi: 10.1016/0272-0590(87)90044-3. [DOI] [PubMed] [Google Scholar]
- Morrow P. E. Possible mechanisms to explain dust overloading of the lungs. Fundam Appl Toxicol. 1988 Apr;10(3):369–384. doi: 10.1016/0272-0590(88)90284-9. [DOI] [PubMed] [Google Scholar]
- Oberdörster G., Ferin J., Gelein R., Soderholm S. C., Finkelstein J. Role of the alveolar macrophage in lung injury: studies with ultrafine particles. Environ Health Perspect. 1992 Jul;97:193–199. doi: 10.1289/ehp.97-1519541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberdörster G., Ferin J., Morrow P. E. Volumetric loading of alveolar macrophages (AM): a possible basis for diminished AM-mediated particle clearance. Exp Lung Res. 1992 Jan-Mar;18(1):87–104. doi: 10.3109/01902149209020653. [DOI] [PubMed] [Google Scholar]
- Piguet P. F., Collart M. A., Grau G. E., Sappino A. P., Vassalli P. Requirement of tumour necrosis factor for development of silica-induced pulmonary fibrosis. Nature. 1990 Mar 15;344(6263):245–247. doi: 10.1038/344245a0. [DOI] [PubMed] [Google Scholar]


