Abstract
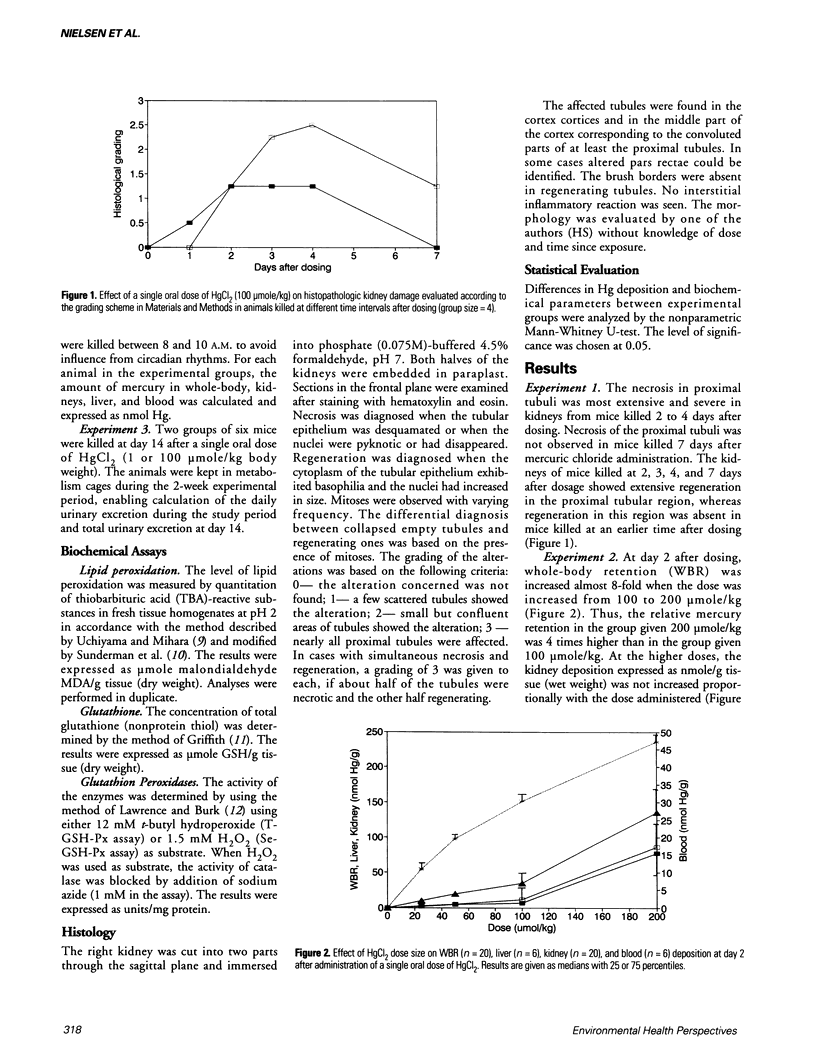
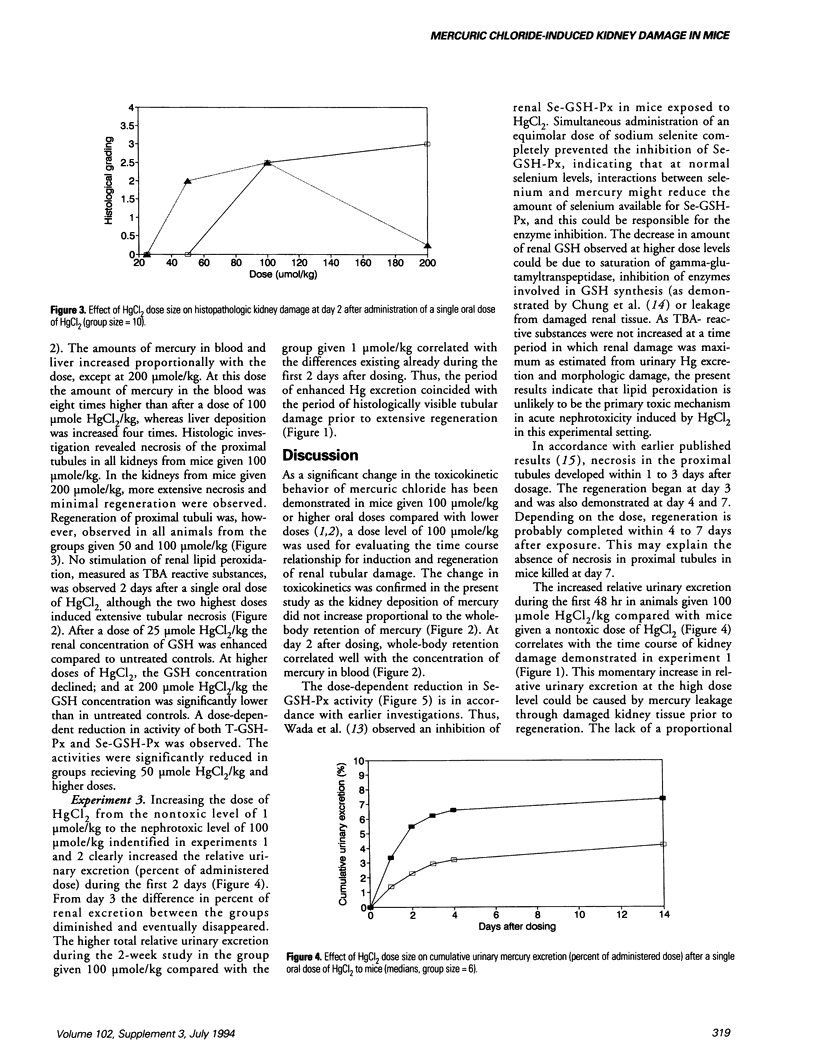
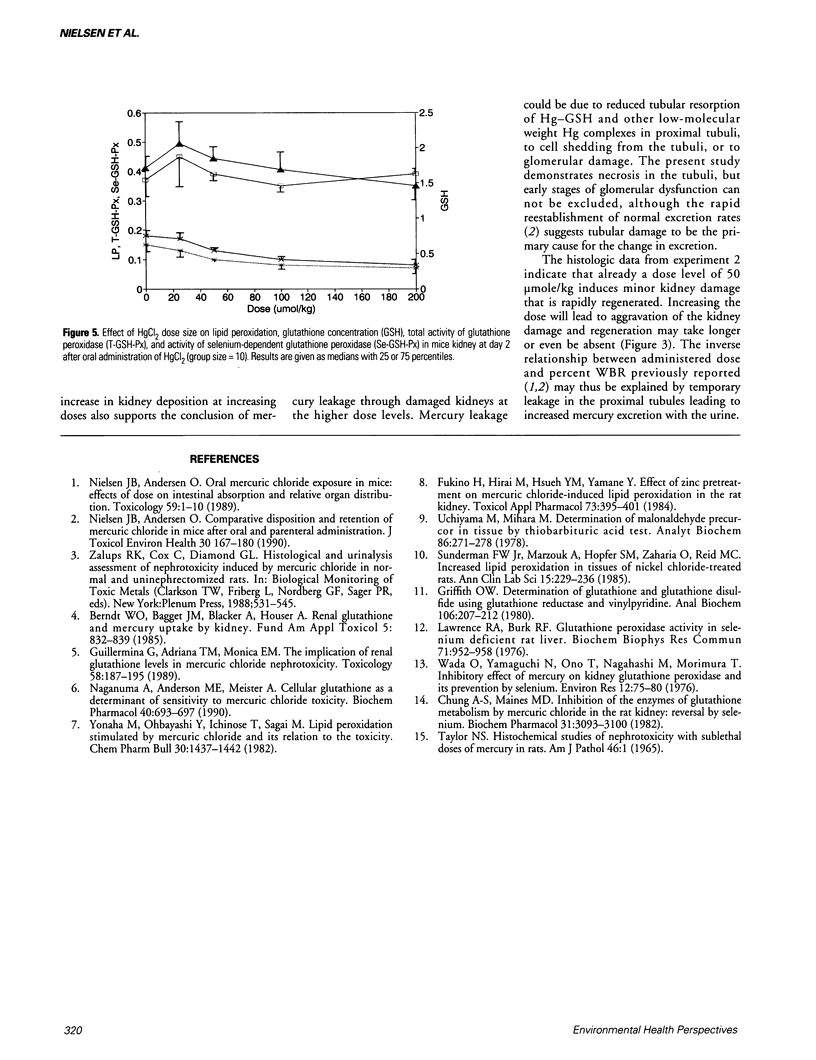
Mercuric chloride is a well-known human and animal nephrotoxicant. Previous studies have demonstrated an inverse relationship between dose size and relative whole-body retention of mercury after oral administration of mercuric chloride to mice. The present study indicates that this inverse relationship is caused by a dose-related induction of kidney damage leading to increasing leakage of mercury through the kidneys. Histopathologic investigation revealed extensive necrosis of the proximal tubules in kidneys from mice exposed to 100 mumole HgCl2/kg or higher doses. Moreover, maximum renal damage occurred between days 2 and 3 after administration. The renal damage was followed by regeneration, which was observed between days 3 and 7 at increasing dose levels up to 100 mumole HgCl2/kg. The amount of glutathione and the glutathione peroxidase activity in kidney decreased with increasing doses of mercuric chloride. The reduced glutathione peroxidase activity was due to a reduction in selenium-dependent glutathione peroxidase activity. The level of lipid peroxidation was not changed by increasing doses of mercuric chloride, and hence was not a primary toxic mechanism in acute nephrotoxicity induced by mercuric chloride.
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ator N. A., Merigan W. H., Jr, McIntire R. W. The effects of brief exposures to carbon monoxide on temporally differentiated responding. Environ Res. 1976 Aug;12(1):75–80. doi: 10.1016/0013-9351(76)90011-6. [DOI] [PubMed] [Google Scholar]
- Berndt W. O., Baggett J. M., Blacker A., Houser M. Renal glutathione and mercury uptake by kidney. Fundam Appl Toxicol. 1985 Oct;5(5):832–839. doi: 10.1016/0272-0590(85)90166-6. [DOI] [PubMed] [Google Scholar]
- Chung A. S., Maines M. D., Reynolds W. A. Inhibition of the enzymes of glutathione metabolism by mercuric chloride in the rat kidney: reversal by selenium. Biochem Pharmacol. 1982 Oct 1;31(19):3093–3100. doi: 10.1016/0006-2952(82)90085-5. [DOI] [PubMed] [Google Scholar]
- Fukino H., Hirai M., Hsueh Y. M., Yamane Y. Effect of zinc pretreatment on mercuric chloride-induced lipid peroxidation in the rat kidney. Toxicol Appl Pharmacol. 1984 May;73(3):395–401. doi: 10.1016/0041-008x(84)90091-7. [DOI] [PubMed] [Google Scholar]
- Griffith O. W. Determination of glutathione and glutathione disulfide using glutathione reductase and 2-vinylpyridine. Anal Biochem. 1980 Jul 15;106(1):207–212. doi: 10.1016/0003-2697(80)90139-6. [DOI] [PubMed] [Google Scholar]
- Guillermina G., Adriana T. M., Monica E. M. The implication of renal glutathione levels in mercuric chloride nephrotoxicity. Toxicology. 1989 Oct 2;58(2):187–195. doi: 10.1016/0300-483x(89)90008-5. [DOI] [PubMed] [Google Scholar]
- Lawrence R. A., Burk R. F. Glutathione peroxidase activity in selenium-deficient rat liver. Biochem Biophys Res Commun. 1976 Aug 23;71(4):952–958. doi: 10.1016/0006-291x(76)90747-6. [DOI] [PubMed] [Google Scholar]
- Mihara M., Uchiyama M. Determination of malonaldehyde precursor in tissues by thiobarbituric acid test. Anal Biochem. 1978 May;86(1):271–278. doi: 10.1016/0003-2697(78)90342-1. [DOI] [PubMed] [Google Scholar]
- Naganuma A., Anderson M. E., Meister A. Cellular glutathione as a determinant of sensitivity to mercuric chloride toxicity. Prevention of toxicity by giving glutathione monoester. Biochem Pharmacol. 1990 Aug 15;40(4):693–697. doi: 10.1016/0006-2952(90)90303-3. [DOI] [PubMed] [Google Scholar]
- Nielsen J. B., Andersen O. Disposition and retention of mercuric chloride in mice after oral and parenteral administration. J Toxicol Environ Health. 1990 Jul;30(3):167–180. doi: 10.1080/15287399009531420. [DOI] [PubMed] [Google Scholar]
- Nielsen J. B., Andersen O. Oral mercuric chloride exposure in mice: effects of dose on intestinal absorption and relative organ distribution. Toxicology. 1989 Nov;59(1):1–10. doi: 10.1016/0300-483x(89)90152-2. [DOI] [PubMed] [Google Scholar]
- Sunderman F. W., Jr, Marzouk A., Hopfer S. M., Zaharia O., Reid M. C. Increased lipid peroxidation in tissues of nickel chloride-treated rats. Ann Clin Lab Sci. 1985 May-Jun;15(3):229–236. [PubMed] [Google Scholar]
- TAYLOR N. S. HISTOCHEMICAL STUDIES OF NEPHROTOXICITY WITH SUBLETHAL DOSES OF MERCURY IN RATS. Am J Pathol. 1965 Jan;46:1–21. [PMC free article] [PubMed] [Google Scholar]
- Yonaha M., Ohbayashi Y., Ichinose T., Sagai M. Lipid peroxidation stimulated by mercuric chloride and its relations to the toxicity. Chem Pharm Bull (Tokyo) 1982 Apr;30(4):1437–1442. doi: 10.1248/cpb.30.1437. [DOI] [PubMed] [Google Scholar]


