Abstract
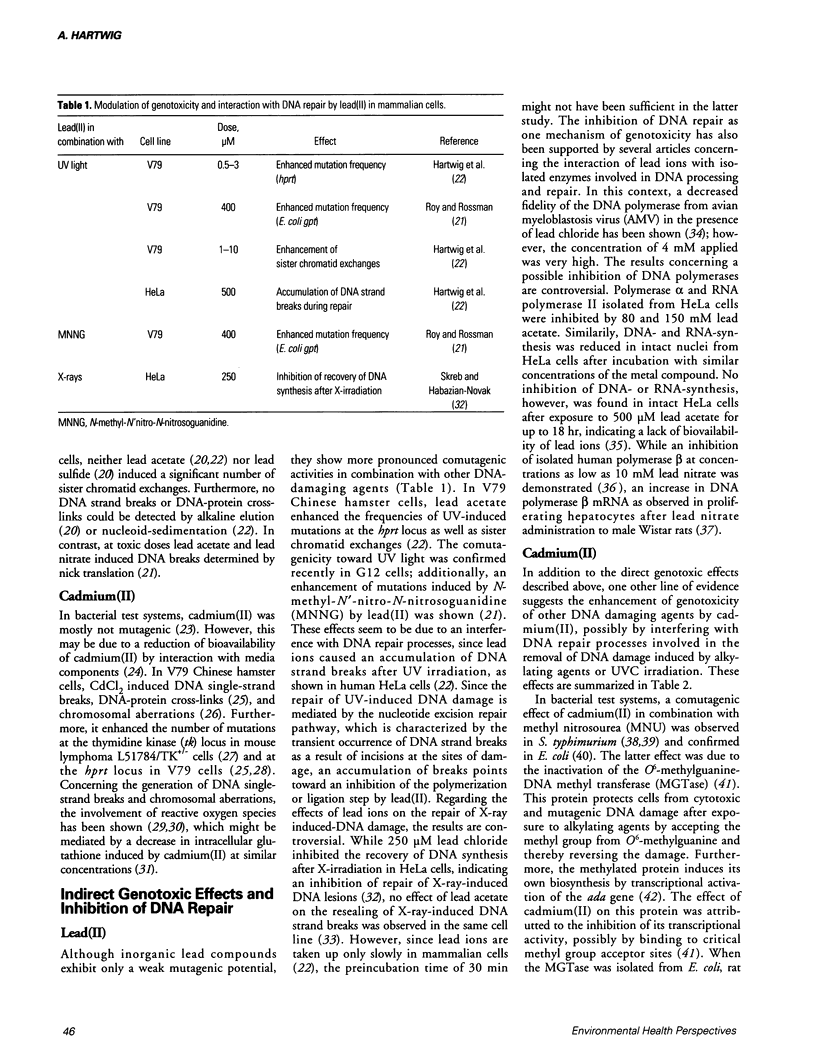
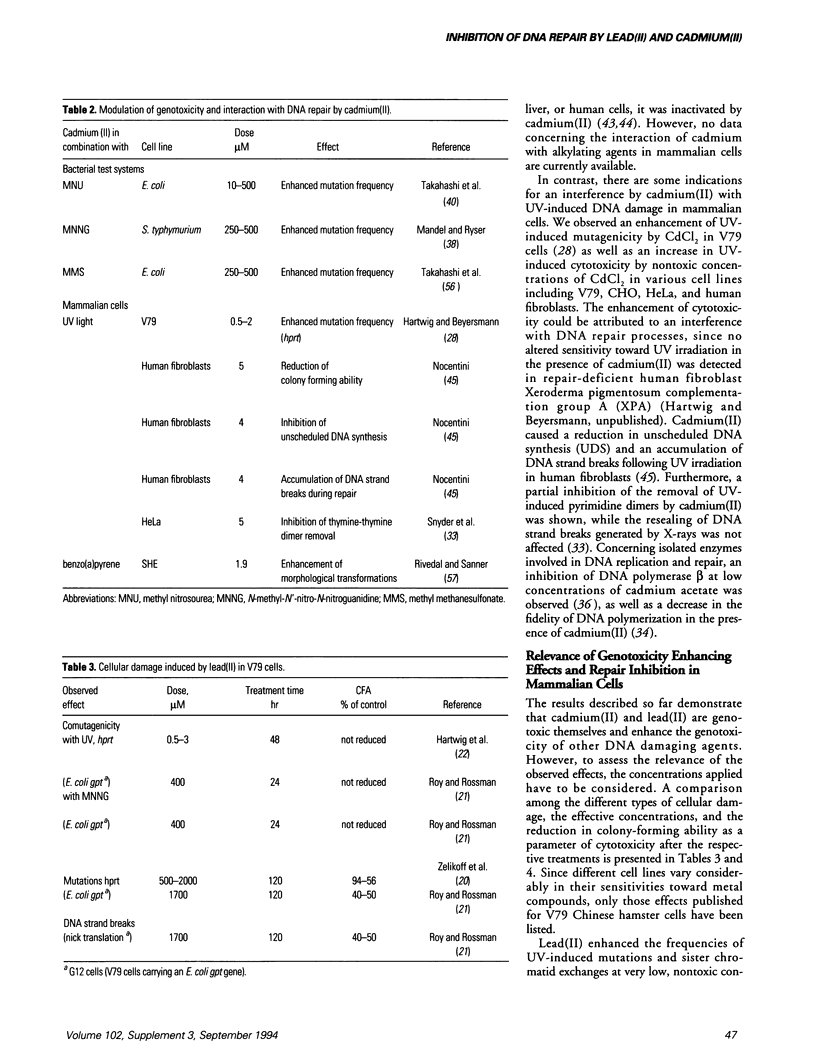
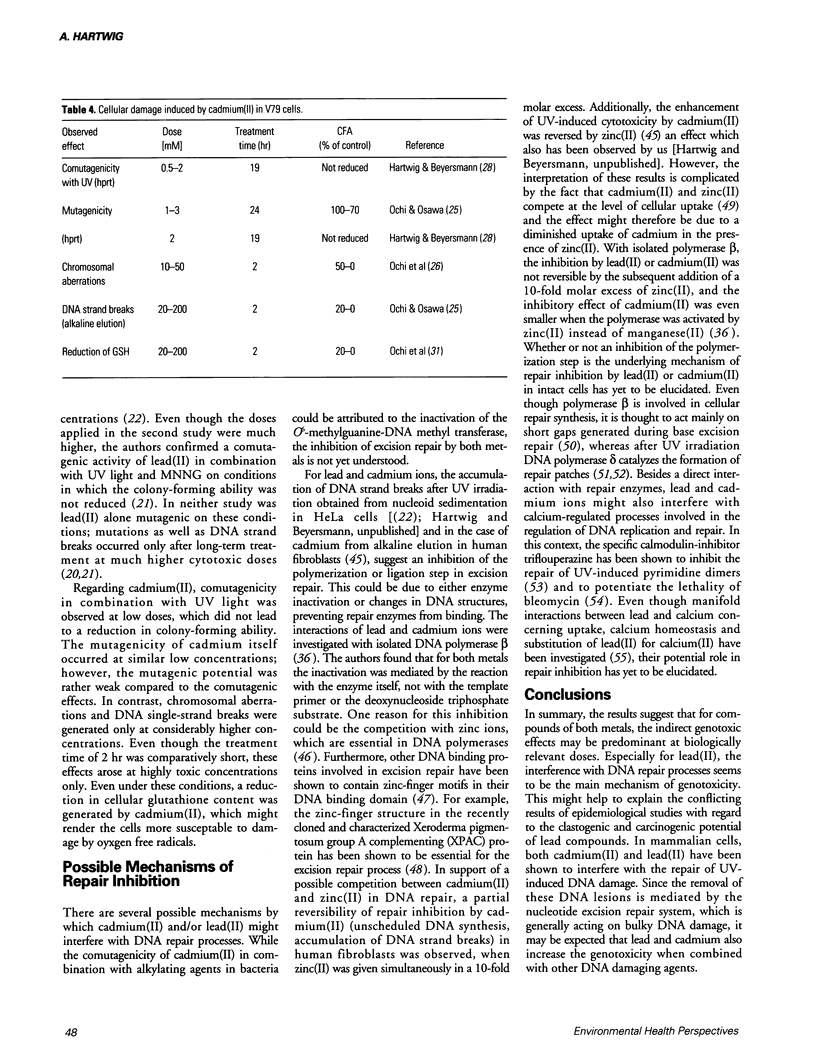
Compounds of lead and cadmium have been shown to be carcinogenic to humans and experimental animals. However, the underlying mechanisms are still not understood. In mammalian cells in culture, lead(II) is weakly mutagenic after long incubation times and generates DNA strand breaks only after treatment with high, toxic doses. Cadmium(II) induces DNA strand breaks and chromosomal aberrations, but its mutagenic potential is rather weak. However, both metals exert pronounced indirect genotoxic effects. Lead(II) is comutagenic towards UV and N-methyl-N-nitro-N-nitrosoguanidine (MNNG) and enhances the number of UV-induced sister chromatid exchanges in V79 Chinese hamster cells. With regard to DNA repair, lead(II) causes an accumulation of DNA strand breaks after UV-irradiation in HeLa cells, indicating an interference with the polymerization or ligation step in excision repair. Cadmium(II) enhances the mutagenicity of UV light in V79 Chinese hamster cells and an increased sensitivity toward UV light is observed in various rodent and human cell lines. Furthermore, an inhibition of unscheduled DNA synthesis after UV-irradiation and a partial inhibition of the removal of UV-induced DNA lesions has been shown. For both metals, the indirect genotoxic effects are observed at low, nontoxic concentrations, suggesting that an interference with DNA repair processes may be predominant at biologically relevant concentrations. This might also explain the conflicting results of epidemiological studies obtained for both metals. Possible mechanisms of repair inhibition are discussed.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bhattacharyya D., Boulden A. M., Foote R. S., Mitra S. Effect of polyvalent metal ions on the reactivity of human O6-methylguanine-DNA methyltransferase. Carcinogenesis. 1988 Apr;9(4):683–685. doi: 10.1093/carcin/9.4.683. [DOI] [PubMed] [Google Scholar]
- Chafouleas J. G., Bolton W. E., Means A. R. Potentiation of bleomycin lethality by anticalmodulin drugs: a role for calmodulin in DNA repair. Science. 1984 Jun 22;224(4655):1346–1348. doi: 10.1126/science.6203171. [DOI] [PubMed] [Google Scholar]
- Charp P. A., Regan J. D. Inhibition of DNA repair by trifluoperazine. Biochim Biophys Acta. 1985 Jan 29;824(1):34–39. doi: 10.1016/0167-4781(85)90026-0. [DOI] [PubMed] [Google Scholar]
- Coleman J. E. Zinc proteins: enzymes, storage proteins, transcription factors, and replication proteins. Annu Rev Biochem. 1992;61:897–946. doi: 10.1146/annurev.bi.61.070192.004341. [DOI] [PubMed] [Google Scholar]
- Cooper W. C., Gaffey W. R. Mortality of lead workers. J Occup Med. 1975 Feb;17(2):100–107. doi: 10.1097/00043764-197502000-00012. [DOI] [PubMed] [Google Scholar]
- DINGWALL-FORDYCE I., LANE R. E. A FOLLOW-UP STUDY OF LEAD WORKERS. Br J Ind Med. 1963 Oct;20:313–315. doi: 10.1136/oem.20.4.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deknudt G., Léonard A. Cytogenetic investigations on leucocytes of workers from a cadmium plant. Environ Physiol Biochem. 1975;5(5):319–327. [PubMed] [Google Scholar]
- Dresler S. L., Gowans B. J., Robinson-Hill R. M., Hunting D. J. Involvement of DNA polymerase delta in DNA repair synthesis in human fibroblasts at late times after ultraviolet irradiation. Biochemistry. 1988 Aug 23;27(17):6379–6383. doi: 10.1021/bi00417a028. [DOI] [PubMed] [Google Scholar]
- Forni A., Sciame' A., Bertazzi P. A., Alessio L. Chromosome and biochemical studies in women occupationally exposed to lead. Arch Environ Health. 1980 May-Jun;35(3):139–146. doi: 10.1080/00039896.1980.10667481. [DOI] [PubMed] [Google Scholar]
- Frenkel G. D., Middleton C. Effects of lead acetate on DNA and RNA synthesis by intact HeLa cells, isolated nuclei and purified polymerases. Biochem Pharmacol. 1987 Jan 15;36(2):265–268. doi: 10.1016/0006-2952(87)90699-x. [DOI] [PubMed] [Google Scholar]
- Hartwig A., Beyersmann D. Comutagenicity and inhibition of DNA repair by metal ions in mammalian cells. Biol Trace Elem Res. 1989 Jul-Sep;21:359–365. doi: 10.1007/BF02917276. [DOI] [PubMed] [Google Scholar]
- Hartwig A., Schlepegrell R., Beyersmann D. Indirect mechanism of lead-induced genotoxicity in cultured mammalian cells. Mutat Res. 1990 May;241(1):75–82. doi: 10.1016/0165-1218(90)90110-n. [DOI] [PubMed] [Google Scholar]
- Kolonel L. N. Association of cadmium with renal cancer. Cancer. 1976 Apr;37(4):1782–1787. doi: 10.1002/1097-0142(197604)37:4<1782::aid-cncr2820370424>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- Lemen R. A., Lee J. S., Wagoner J. K., Blejer H. P. Cancer mortality among cadmium production workers. Ann N Y Acad Sci. 1976;271:273–279. doi: 10.1111/j.1749-6632.1976.tb23122.x. [DOI] [PubMed] [Google Scholar]
- Lindahl T., Sedgwick B., Sekiguchi M., Nakabeppu Y. Regulation and expression of the adaptive response to alkylating agents. Annu Rev Biochem. 1988;57:133–157. doi: 10.1146/annurev.bi.57.070188.001025. [DOI] [PubMed] [Google Scholar]
- Mandel R., Ryser H. J. Mechanisms of synergism in the mutagenicity of cadmium and N-methyl-N-nitrosourea in Salmonella typhimurium: the effect of pH. Mutat Res. 1987 Jan;176(1):1–10. doi: 10.1016/0027-5107(87)90246-6. [DOI] [PubMed] [Google Scholar]
- Mandel R., Ryser H. J. Mutagenicity of cadmium in Salmonella typhimurium and its synergism with two nitrosamines. Mutat Res. 1984 Oct;138(1):9–16. doi: 10.1016/0165-1218(84)90079-x. [DOI] [PubMed] [Google Scholar]
- Menegazzi M., Carcereri de Prati A., Ogura T., Columbano A., Ledda-Columbano G. M., Libonati M., Esumi H., Suzuki H. Involvement of DNA polymerase beta in proliferation of rat liver induced by lead nitrate or partial hepatectomy. FEBS Lett. 1992 Sep 28;310(2):135–138. doi: 10.1016/0014-5793(92)81314-c. [DOI] [PubMed] [Google Scholar]
- Miyamoto I., Miura N., Niwa H., Miyazaki J., Tanaka K. Mutational analysis of the structure and function of the xeroderma pigmentosum group A complementing protein. Identification of essential domains for nuclear localization and DNA excision repair. J Biol Chem. 1992 Jun 15;267(17):12182–12187. [PubMed] [Google Scholar]
- Nishida C., Reinhard P., Linn S. DNA repair synthesis in human fibroblasts requires DNA polymerase delta. J Biol Chem. 1988 Jan 5;263(1):501–510. [PubMed] [Google Scholar]
- Nordenson I., Beckman L. Occupational and environmental risks in and around a smelter in northern Sweden. VII. Reanalysis and follow-up of chromosomal aberrations in workers exposed to arsenic. Hereditas. 1982;96(2):175–181. doi: 10.1111/j.1601-5223.1982.tb00847.x. [DOI] [PubMed] [Google Scholar]
- Oberly T. J., Piper C. E., McDonald D. S. Mutagenicity of metal salts in the L5178Y mouse lymphoma assay. J Toxicol Environ Health. 1982 Mar;9(3):367–376. doi: 10.1080/15287398209530170. [DOI] [PubMed] [Google Scholar]
- Ochi T., Mogi M., Watanabe M., Ohsawa M. Induction of chromosomal aberrations in cultured Chinese hamster cells by short-term treatment with cadmium chloride. Mutat Res. 1984 Aug-Sep;137(2-3):103–109. doi: 10.1016/0165-1218(84)90098-3. [DOI] [PubMed] [Google Scholar]
- Ochi T., Ohsawa M. Induction of 6-thioguanine-resistant mutants and single-strand scission of DNA by cadmium chloride in cultured Chinese hamster cells. Mutat Res. 1983 Sep;111(1):69–78. doi: 10.1016/0027-5107(83)90009-x. [DOI] [PubMed] [Google Scholar]
- Ochi T., Ohsawa M. Participation of active oxygen species in the induction of chromosomal aberrations by cadmium chloride in cultured Chinese hamster cells. Mutat Res. 1985 Jul;143(3):137–142. doi: 10.1016/s0165-7992(85)80024-5. [DOI] [PubMed] [Google Scholar]
- Ochi T., Takahashi K., Ohsawa M. Indirect evidence for the induction of a prooxidant state by cadmium chloride in cultured mammalian cells and a possible mechanism for the induction. Mutat Res. 1987 Oct;180(2):257–266. doi: 10.1016/0027-5107(87)90222-3. [DOI] [PubMed] [Google Scholar]
- POTTS C. L. CADMIUM PROTEINURIA--THE HEALTH OF BATTERY WORKERS EXPOSED TO CADMIUM OXIDE DUST. Ann Occup Hyg. 1965 Mar;8:55–61. doi: 10.1093/annhyg/8.1.55. [DOI] [PubMed] [Google Scholar]
- Pagano D. A., Zeiger E. Conditions for detecting the mutagenicity of divalent metals in Salmonella typhimurium. Environ Mol Mutagen. 1992;19(2):139–146. doi: 10.1002/em.2850190208. [DOI] [PubMed] [Google Scholar]
- Popenoe E. A., Schmaeler M. A. Interaction of human DNA polymerase beta with ions of copper, lead, and cadmium. Arch Biochem Biophys. 1979 Aug;196(1):109–120. doi: 10.1016/0003-9861(79)90557-5. [DOI] [PubMed] [Google Scholar]
- Pounds J. G. Effect of lead intoxication on calcium homeostasis and calcium-mediated cell function: a review. Neurotoxicology. 1984 Fall;5(3):295–331. [PubMed] [Google Scholar]
- Rivedal E., Sanner T. Metal salts as promoters of in vitro morphological transformation of hamster embryo cells initiated by benzo(a)pyrene. Cancer Res. 1981 Jul;41(7):2950–2953. [PubMed] [Google Scholar]
- Roy N. K., Rossman T. G. Mutagenesis and comutagenesis by lead compounds. Mutat Res. 1992 Dec;298(2):97–103. doi: 10.1016/0165-1218(92)90034-w. [DOI] [PubMed] [Google Scholar]
- Scicchitano D. A., Pegg A. E. Inhibition of O6-alkylguanine-DNA-alkyltransferase by metals. Mutat Res. 1987 Nov;192(3):207–210. doi: 10.1016/0165-7992(87)90057-1. [DOI] [PubMed] [Google Scholar]
- Shirai T., Ohshima M., Masuda A., Tamano S., Ito N. Promotion of 2-(ethylnitrosamino)ethanol-induced renal carcinogenesis in rats by nephrotoxic compounds: positive responses with folic acid, basic lead acetate, and N-(3,5-dichlorophenyl)succinimide but not with 2,3-dibromo-1-propanol phosphate. J Natl Cancer Inst. 1984 Feb;72(2):477–482. [PubMed] [Google Scholar]
- Sirover M. A., Loeb L. A. Metal activation of DNA synthesis. Biochem Biophys Res Commun. 1976 Jun 7;70(3):812–817. doi: 10.1016/0006-291x(76)90664-1. [DOI] [PubMed] [Google Scholar]
- Snyder R. D., Davis G. F., Lachmann P. J. Inhibition by metals of X-ray and ultraviolet-induced DNA repair in human cells. Biol Trace Elem Res. 1989 Jul-Sep;21:389–398. doi: 10.1007/BF02917280. [DOI] [PubMed] [Google Scholar]
- Snyder R. D. Role of active oxygen species in metal-induced DNA strand breakage in human diploid fibroblasts. Mutat Res. 1988 May;193(3):237–246. doi: 10.1016/0167-8817(88)90034-x. [DOI] [PubMed] [Google Scholar]
- Stayner L., Smith R., Thun M., Schnorr T., Lemen R. A dose-response analysis and quantitative assessment of lung cancer risk and occupational cadmium exposure. Ann Epidemiol. 1992 May;2(3):177–194. doi: 10.1016/1047-2797(92)90052-r. [DOI] [PubMed] [Google Scholar]
- Takahashi K., Imaeda T., Kawazoe Y. Effect of metal ions on the adaptive response induced by N-methyl-N-nitrosourea in Escherichia coli. Biochem Biophys Res Commun. 1988 Dec 30;157(3):1124–1130. doi: 10.1016/s0006-291x(88)80990-2. [DOI] [PubMed] [Google Scholar]
- Takahashi K., Imaeda T., Kawazoe Y. Inhibitory effect of cadmium and mercury ions on induction of the adaptive response in Escherichia coli. Mutat Res. 1991 Jan;254(1):45–53. doi: 10.1016/0921-8777(91)90039-r. [DOI] [PubMed] [Google Scholar]
- Takahashi K., Suzuki M., Sekiguchi M., Kawazoe Y. Effect of metal ions on transcription of the ada gene which encodes O6-methylguanine-DNA methyltransferase of Escherichia coli. Chem Pharm Bull (Tokyo) 1992 Sep;40(9):2483–2486. doi: 10.1248/cpb.40.2483. [DOI] [PubMed] [Google Scholar]
- Tanner D. C., Lipsky M. M. Effect of lead acetate on N-(4'-fluoro-4-biphenyl)acetamide-induced renal carcinogenesis in the rat. Carcinogenesis. 1984 Sep;5(9):1109–1113. doi: 10.1093/carcin/5.9.1109. [DOI] [PubMed] [Google Scholar]
- Thun M. J., Schnorr T. M., Smith A. B., Halperin W. E., Lemen R. A. Mortality among a cohort of U.S. cadmium production workers--an update. J Natl Cancer Inst. 1985 Feb;74(2):325–333. [PubMed] [Google Scholar]
- Wade G. G., Mandel R., Ryser H. J. Marked synergism of dimethylnitrosamine carcinogenesis in rats exposed to cadmium. Cancer Res. 1987 Dec 15;47(24 Pt 1):6606–6613. [PubMed] [Google Scholar]
- Zelikoff J. T., Li J. H., Hartwig A., Wang X. W., Costa M., Rossman T. G. Genetic toxicology of lead compounds. Carcinogenesis. 1988 Oct;9(10):1727–1732. doi: 10.1093/carcin/9.10.1727. [DOI] [PubMed] [Google Scholar]


