Abstract
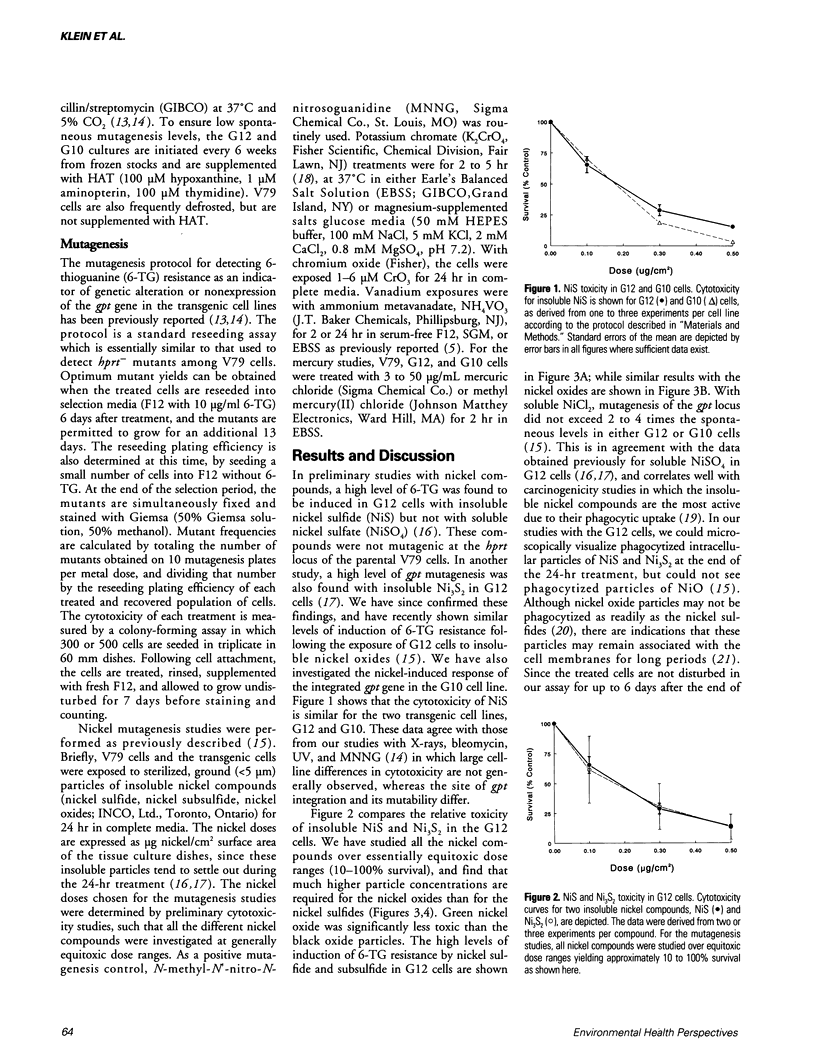
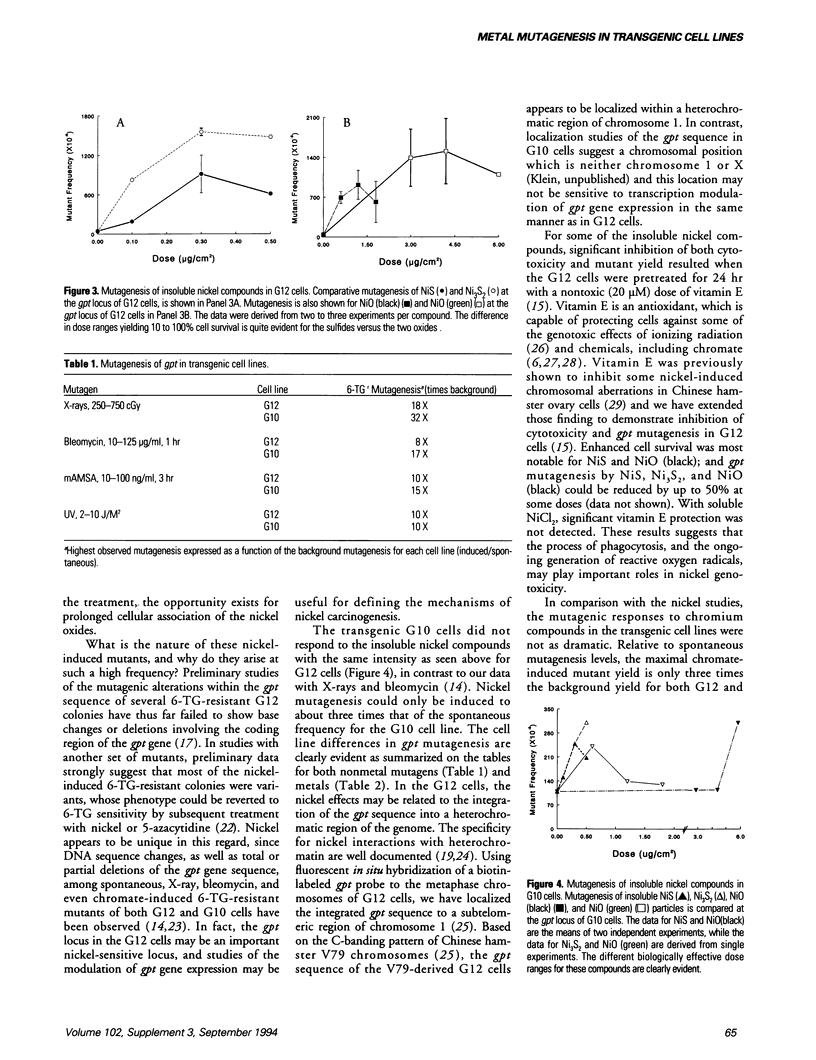
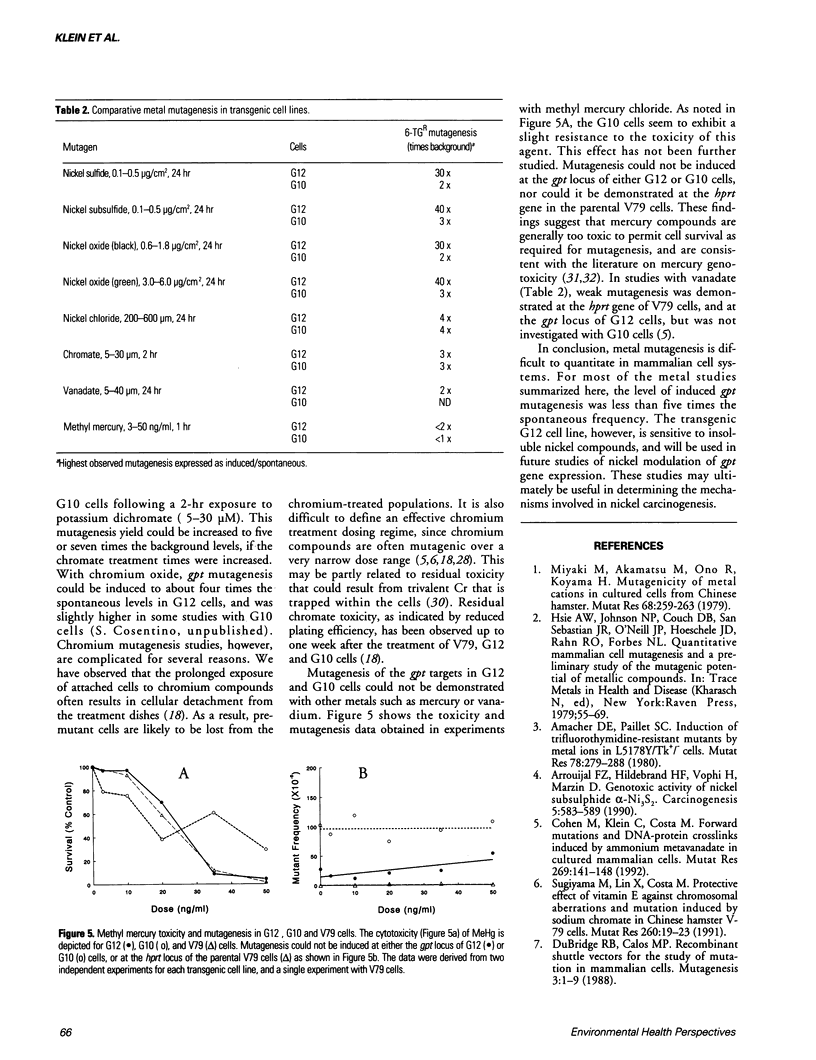
Metals are toxic agents for which genotoxic effects are often difficult to demonstrate. To study metal mutagenesis, we have used two stable hprt/gpt+ transgenic cell lines that were derived from Chinese hamster V79 cells. Both the G12 and G10 cell lines are known to be very sensitive to clastogens such as X-rays and bleomycin, with the mutagenic response of the integrated xanthine guanine phosphoribosyl transferase (gpt) gene in G10 usually exceeding that of the same gene in the transgenic G12 cells. In studies with carcinogenic insoluble nickel compounds, a high level of mutagenesis was found at the gpt locus of G12 cells but not at the endogenous hypoxanthine phosphoribosyl transferase (hprt) locus of V79 cells. We have since demonstrated the similar recovery of a high frequency of viable G12 mutants with other insoluble nickel salts including nickel oxides (black and green). The relative mutant yield for the insoluble nickel compounds (G12 > G10) is the opposite of that obtained with nonmetal clastogens (G10 > G12). In the G12 cells, nickel mutagenesis may be related to the integration of the gpt sequence into a heterochromatic region of the genome. For some of the insoluble nickel compounds, significant inhibition of both cytotoxicity and mutant yield resulted when the G12 cells were pretreated with vitamin E. In comparison with the nickel studies, the mutagenic responses to chromium compounds in these cell lines were not as dramatic. Mutagenesis of the gpt target could not be demonstrated with other metals such as mercury or vanadium.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amacher D. E., Paillet S. C. Induction of trifluorothymidine-resistant mutants by metal ions in L5178Y/TK+/- cells. Mutat Res. 1980 Jul;78(3):279–288. doi: 10.1016/0165-1218(80)90110-x. [DOI] [PubMed] [Google Scholar]
- Arrouijal F. Z., Hildebrand H. F., Vophi H., Marzin D. Genotoxic activity of nickel subsulphide alpha-Ni3S2. Mutagenesis. 1990 Nov;5(6):583–589. doi: 10.1093/mutage/5.6.583. [DOI] [PubMed] [Google Scholar]
- Ashman C. R. Retroviral shuttle vectors as a tool for the study of mutational specificity (base substitution/deletion/mutational hotspot). Mutat Res. 1989 Mar-May;220(2-3):143–149. doi: 10.1016/0165-1110(89)90020-1. [DOI] [PubMed] [Google Scholar]
- Betti C., Davini T., Barale R. Genotoxic activity of methyl mercury chloride and dimethyl mercury in human lymphocytes. Mutat Res. 1992 Apr;281(4):255–260. doi: 10.1016/0165-7992(92)90018-d. [DOI] [PubMed] [Google Scholar]
- Cohen M. D., Klein C. B., Costa M. Forward mutations and DNA-protein crosslinks induced by ammonium metavanadate in cultured mammalian cells. Mutat Res. 1992 Sep;269(1):141–148. doi: 10.1016/0027-5107(92)90169-3. [DOI] [PubMed] [Google Scholar]
- Costa M. Molecular mechanisms of nickel carcinogenesis. Annu Rev Pharmacol Toxicol. 1991;31:321–337. doi: 10.1146/annurev.pa.31.040191.001541. [DOI] [PubMed] [Google Scholar]
- Debenham P. G., Webb M. B., Stretch A., Thacker J. Examination of vectors with two dominant, selectable genes for DNA repair and mutation studies in mammalian cells. Mutat Res. 1988 May;199(1):145–158. doi: 10.1016/0027-5107(88)90240-0. [DOI] [PubMed] [Google Scholar]
- DuBridge R. B., Calos M. P. Recombinant shuttle vectors for the study of mutation in mammalian cells. Mutagenesis. 1988 Jan;3(1):1–9. doi: 10.1093/mutage/3.1.1. [DOI] [PubMed] [Google Scholar]
- Kargacin B., Klein C. B., Costa M. Mutagenic responses of nickel oxides and nickel sulfides in Chinese hamster V79 cell lines at the xanthine-guanine phosphoribosyl transferase locus. Mutat Res. 1993 Jun;300(1):63–72. doi: 10.1016/0165-1218(93)90141-y. [DOI] [PubMed] [Google Scholar]
- Klein C. B., Rossman T. G. Transgenic Chinese hamster V79 cell lines which exhibit variable levels of gpt mutagenesis. Environ Mol Mutagen. 1990;16(1):1–12. doi: 10.1002/em.2850160102. [DOI] [PubMed] [Google Scholar]
- Klein C. B., Su L., Rossman T. G., Snow E. T. Transgenic gpt+ V79 cell lines differ in their mutagenic response to clastogens. Mutat Res. 1994 Jan 16;304(2):217–228. doi: 10.1016/0027-5107(94)90214-3. [DOI] [PubMed] [Google Scholar]
- Lin X. H., Sugiyama M., Costa M. Differences in the effect of vitamin E on nickel sulfide or nickel chloride-induced chromosomal aberrations in mammalian cells. Mutat Res. 1991 Jun;260(2):159–164. doi: 10.1016/0165-1218(91)90004-6. [DOI] [PubMed] [Google Scholar]
- Léonard A., Jacquet P., Lauwerys R. R. Mutagenicity and teratogenicity of mercury compounds. Mutat Res. 1983 Jan;114(1):1–18. doi: 10.1016/0165-1110(83)90017-9. [DOI] [PubMed] [Google Scholar]
- Miura T., Patierno S. R., Sakuramoto T., Landolph J. R. Morphological and neoplastic transformation of C3H/10T1/2 Cl 8 mouse embryo cells by insoluble carcinogenic nickel compounds. Environ Mol Mutagen. 1989;14(2):65–78. doi: 10.1002/em.2850140202. [DOI] [PubMed] [Google Scholar]
- Miyaki M., Akamatsu N., Ono T., Koyama H. Mutagenicity of metal cations in cultured cells from Chinese hamster. Mutat Res. 1979 Nov;68(3):259–263. doi: 10.1016/0165-1218(79)90157-5. [DOI] [PubMed] [Google Scholar]
- Radner B. S., Kennedy A. R. Suppression of X-ray induced transformation by vitamin E in mouse C3H/10T1/2 cells. Cancer Lett. 1986 Jul;32(1):25–32. doi: 10.1016/0304-3835(86)90035-2. [DOI] [PubMed] [Google Scholar]
- Romac S., Leong P., Sockett H., Hutchinson F. DNA base sequence changes induced by ultraviolet light mutagenesis of a gene on a chromosome in Chinese hamster ovary cells. J Mol Biol. 1989 Sep 20;209(2):195–204. doi: 10.1016/0022-2836(89)90272-6. [DOI] [PubMed] [Google Scholar]
- Sen P., Costa M. Induction of chromosomal damage in Chinese hamster ovary cells by soluble and particulate nickel compounds: preferential fragmentation of the heterochromatic long arm of the X-chromosome by carcinogenic crystalline NiS particles. Cancer Res. 1985 May;45(5):2320–2325. [PubMed] [Google Scholar]
- Sugiyama M., Ando A., Furuno A., Furlong N. B., Hidaka T., Ogura R. Effects of vitamin E, vitamin B2 and selenite on DNA single strand breaks induced by sodium chromate (VI). Cancer Lett. 1987 Dec;38(1-2):1–7. doi: 10.1016/0304-3835(87)90193-5. [DOI] [PubMed] [Google Scholar]
- Sugiyama M., Ando A., Ogura R. Effect of vitamin E on survival, glutathione reductase and formation of chromium (V) in Chinese hamster V-79 cells treated with sodium chromate (VI). Carcinogenesis. 1989 Apr;10(4):737–741. doi: 10.1093/carcin/10.4.737. [DOI] [PubMed] [Google Scholar]
- Sugiyama M., Lin X. H., Costa M. Protective effect of vitamin E against chromosomal aberrations and mutation induced by sodium chromate in Chinese hamster V79 cells. Mutat Res. 1991 May;260(1):19–23. doi: 10.1016/0165-1218(91)90076-x. [DOI] [PubMed] [Google Scholar]
- Sunderman F. W., Jr, Hopfer S. M., Knight J. A., McCully K. S., Cecutti A. G., Thornhill P. G., Conway K., Miller C., Patierno S. R., Costa M. Physicochemical characteristics and biological effects of nickel oxides. Carcinogenesis. 1987 Feb;8(2):305–313. doi: 10.1093/carcin/8.2.305. [DOI] [PubMed] [Google Scholar]
- Thacker J. The molecular nature of mutations in cultured mammalian cells: a review. Mutat Res. 1985 Jun-Jul;150(1-2):431–442. doi: 10.1016/0027-5107(85)90140-x. [DOI] [PubMed] [Google Scholar]
- Tindall K. R., Stankowski L. F., Jr Molecular analysis of spontaneous mutations at the gpt locus in Chinese hamster ovary (AS52) cells. Mutat Res. 1989 Mar-May;220(2-3):241–253. doi: 10.1016/0165-1110(89)90028-6. [DOI] [PubMed] [Google Scholar]


