Abstract
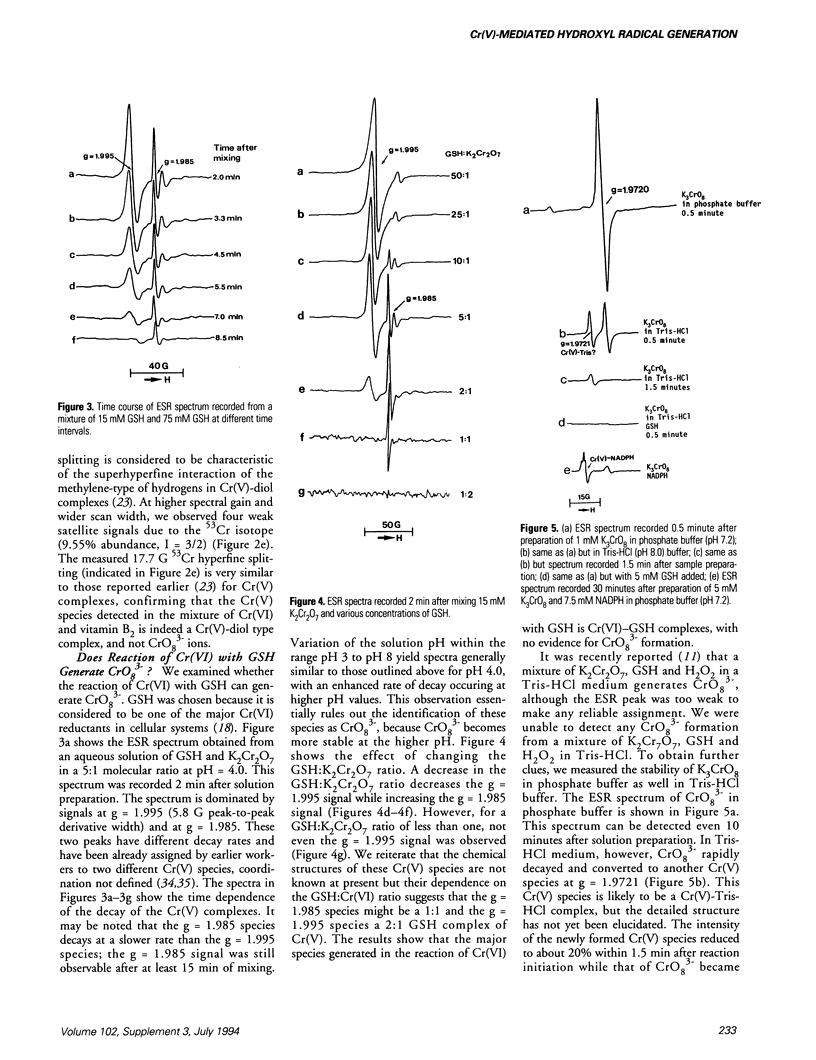
While Cr(V) species and .OH radicals have been suggested to play significant roles in the mechanism of chromate-related carcinogenesis, controversy still exists regarding the identity of the Cr(V) species and their role in the generation of .OH radicals. Some recent studies have suggested that the primary Cr(V) species involved is the tetraperoxochromate(V) (CrO8(3-)) ion, which produces .OH radical either on decomposition or by reaction with H2O2. The present study utilized ESR and spin trapping techniques to probe this mechanism. The results obtained show that (i) CrO8(3-) is not formed in any significant quantity in the reaction of chromate with biologically relevant reductants such as glutathione, glutathione reductase, NAD(P)H, ascorbate, vitamin B2, etc. (ii) Decomposition of CrO8(3-), or its reaction with H2O2 does not generate any significant amount of .OH radicals. (iii) The major Cr(V) species formed are complexes of Cr(V) with reductant moieties as ligands. (iv) These Cr(V) complexes generate .OH radicals from H2O2 via Fenton-like reaction. The present study thus disagrees with the recently proposed "tetraperoxochromate(V) theory of carcinogenesis from chromate." Instead, it suggests an alternative mechanism, which might be labeled as "the Cr(V)-complexation-Fenton reaction model of carcinogenesis from chromate.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aiyar J., Berkovits H. J., Floyd R. A., Wetterhahn K. E. Reaction of chromium (VI) with hydrogen peroxide in the presence of glutathione: reactive intermediates and resulting DNA damage. Chem Res Toxicol. 1990 Nov-Dec;3(6):595–603. doi: 10.1021/tx00018a016. [DOI] [PubMed] [Google Scholar]
- Buettner G. R. Spin trapping: ESR parameters of spin adducts. Free Radic Biol Med. 1987;3(4):259–303. doi: 10.1016/s0891-5849(87)80033-3. [DOI] [PubMed] [Google Scholar]
- Costa M. DNA-protein complexes induced by chromate and other carcinogens. Environ Health Perspect. 1991 May;92:45–52. doi: 10.1289/ehp.919245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Flora S., Bagnasco M., Serra D., Zanacchi P. Genotoxicity of chromium compounds. A review. Mutat Res. 1990 Mar;238(2):99–172. doi: 10.1016/0165-1110(90)90007-x. [DOI] [PubMed] [Google Scholar]
- Enterline P. E. Respiratory cancer among chromate workers. J Occup Med. 1974 Aug;16(8):523–526. [PubMed] [Google Scholar]
- Graf E., Mahoney J. R., Bryant R. G., Eaton J. W. Iron-catalyzed hydroxyl radical formation. Stringent requirement for free iron coordination site. J Biol Chem. 1984 Mar 25;259(6):3620–3624. [PubMed] [Google Scholar]
- Kasprzak K. S. The role of oxidative damage in metal carcinogenicity. Chem Res Toxicol. 1991 Nov-Dec;4(6):604–615. doi: 10.1021/tx00024a002. [DOI] [PubMed] [Google Scholar]
- Kawanishi S., Inoue S., Sano S. Mechanism of DNA cleavage induced by sodium chromate(VI) in the presence of hydrogen peroxide. J Biol Chem. 1986 May 5;261(13):5952–5958. [PubMed] [Google Scholar]
- Klein C. B., Frenkel K., Costa M. The role of oxidative processes in metal carcinogenesis. Chem Res Toxicol. 1991 Nov-Dec;4(6):592–604. doi: 10.1021/tx00024a001. [DOI] [PubMed] [Google Scholar]
- Kortenkamp A., Ozolins Z., Beyersmann D., O'Brien P. Generation of PM2 DNA breaks in the course of reduction of chromium(VI) by glutathione. Mutat Res. 1989 Feb;216(1):19–26. doi: 10.1016/0165-1161(89)90019-8. [DOI] [PubMed] [Google Scholar]
- Langård S., Norseth T. A cohort study of bronchial carcinomas in workers producing chromate pigments. Br J Ind Med. 1975 Feb;32(1):62–65. doi: 10.1136/oem.32.1.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefebvre Y., Pézerat H. Production of activated species of oxygen during the chromate(VI)-ascorbate reaction: implication in carcinogenesis. Chem Res Toxicol. 1992 Jul-Aug;5(4):461–463. doi: 10.1021/tx00028a002. [DOI] [PubMed] [Google Scholar]
- Léonard A., Lauwerys R. R. Carcinogenicity and mutagenicity of chromium. Mutat Res. 1980 Nov;76(3):227–239. doi: 10.1016/0165-1110(80)90018-4. [DOI] [PubMed] [Google Scholar]
- Meister A., Anderson M. E. Glutathione. Annu Rev Biochem. 1983;52:711–760. doi: 10.1146/annurev.bi.52.070183.003431. [DOI] [PubMed] [Google Scholar]
- Mikalsen A., Alexander J., Andersen R. A., Daae H. L. Reduction of hexavalent chromium in a reconstituted system of cytochrome P-450 and cytochrome b5. Chem Biol Interact. 1989;71(2-3):213–221. doi: 10.1016/0009-2797(89)90036-7. [DOI] [PubMed] [Google Scholar]
- Rossi S. C., Gorman N., Wetterhahn K. E. Mitochondrial reduction of the carcinogen chromate: formation of chromium(V). Chem Res Toxicol. 1988 Mar-Apr;1(2):101–107. doi: 10.1021/tx00002a003. [DOI] [PubMed] [Google Scholar]
- Rossi S. C., Wetterhahn K. E. Chromium(V) is produced upon reduction of chromate by mitochondrial electron transport chain complexes. Carcinogenesis. 1989 May;10(5):913–920. doi: 10.1093/carcin/10.5.913. [DOI] [PubMed] [Google Scholar]
- Shi X. G., Dalal N. S. On the hydroxyl radical formation in the reaction between hydrogen peroxide and biologically generated chromium(V) species. Arch Biochem Biophys. 1990 Mar;277(2):342–350. doi: 10.1016/0003-9861(90)90589-q. [DOI] [PubMed] [Google Scholar]
- Shi X. G., Sun X. L., Gannett P. M., Dalal N. S. Deferoxamine inhibition of Cr(V)-mediated radical generation and deoxyguanine hydroxylation: ESR and HPLC evidence. Arch Biochem Biophys. 1992 Mar;293(2):281–286. doi: 10.1016/0003-9861(92)90396-e. [DOI] [PubMed] [Google Scholar]
- Shi X. L., Dalal N. S. Chromium (V) and hydroxyl radical formation during the glutathione reductase-catalyzed reduction of chromium (VI). Biochem Biophys Res Commun. 1989 Aug 30;163(1):627–634. doi: 10.1016/0006-291x(89)92183-9. [DOI] [PubMed] [Google Scholar]
- Shi X. L., Dalal N. S. ESR spin trapping detection of hydroxyl radicals in the reactions of Cr(V) complexes with hydrogen peroxide. Free Radic Res Commun. 1990;10(1-2):17–26. doi: 10.3109/10715769009145929. [DOI] [PubMed] [Google Scholar]
- Shi X. L., Dalal N. S. Evidence for a Fenton-type mechanism for the generation of .OH radicals in the reduction of Cr(VI) in cellular media. Arch Biochem Biophys. 1990 Aug 15;281(1):90–95. doi: 10.1016/0003-9861(90)90417-w. [DOI] [PubMed] [Google Scholar]
- Shi X. L., Dalal N. S. NADPH-dependent flavoenzymes catalyze one electron reduction of metal ions and molecular oxygen and generate hydroxyl radicals. FEBS Lett. 1990 Dec 10;276(1-2):189–191. doi: 10.1016/0014-5793(90)80539-u. [DOI] [PubMed] [Google Scholar]
- Shi X. L., Dalal N. S. On the mechanism of the chromate reduction by glutathione: ESR evidence for the glutathionyl radical and an isolable Cr(V) intermediate. Biochem Biophys Res Commun. 1988 Oct 14;156(1):137–142. doi: 10.1016/s0006-291x(88)80815-5. [DOI] [PubMed] [Google Scholar]
- Shi X. L., Dalal N. S. One-electron reduction of chromate by NADPH-dependent glutathione reductase. J Inorg Biochem. 1990 Sep;40(1):1–12. doi: 10.1016/0162-0134(90)80034-u. [DOI] [PubMed] [Google Scholar]
- Shi X. L., Dalal N. S. The role of superoxide radical in chromium (VI)-generated hydroxyl radical: the Cr(VI) Haber-Weiss cycle. Arch Biochem Biophys. 1992 Jan;292(1):323–327. doi: 10.1016/0003-9861(92)90085-b. [DOI] [PubMed] [Google Scholar]
- Shi X. L., Dalal N. S., Vallyathan V. One-electron reduction of carcinogen chromate by microsomes, mitochondria, and Escherichia coli: identification of Cr(V) and .OH radical. Arch Biochem Biophys. 1991 Nov 1;290(2):381–386. doi: 10.1016/0003-9861(91)90555-w. [DOI] [PubMed] [Google Scholar]
- Standeven A. M., Wetterhahn K. E. Is there a role for reactive oxygen species in the mechanism of chromium(VI) carcinogenesis? Chem Res Toxicol. 1991 Nov-Dec;4(6):616–625. doi: 10.1021/tx00024a003. [DOI] [PubMed] [Google Scholar]
- Sugiyama M., Ando A., Ogura R. Vitamin B2-enhancement of sodium chromate (VI)--Induced DNA single strand breaks: ESR study of the action of vitamin B2. Biochem Biophys Res Commun. 1989 Mar 31;159(3):1080–1085. doi: 10.1016/0006-291x(89)92219-5. [DOI] [PubMed] [Google Scholar]
- Sugiyama M. Effects of vitamin E and vitamin B2 on chromate-induced DNA lesions. Biol Trace Elem Res. 1989 Jul-Sep;21:399–404. doi: 10.1007/BF02917281. [DOI] [PubMed] [Google Scholar]
- Sugiyama M., Wang X. W., Costa M. Comparison of DNA lesions and cytotoxicity induced by calcium chromate in human, mouse, and hamster cell lines. Cancer Res. 1986 Sep;46(9):4547–4551. [PubMed] [Google Scholar]
- Tsapakos M. J., Wetterhahn K. E. The interaction of chromium with nucleic acids. Chem Biol Interact. 1983 Sep 1;46(2):265–277. doi: 10.1016/0009-2797(83)90034-0. [DOI] [PubMed] [Google Scholar]
- Venitt S., Levy L. S. Mutagenicity of chromates in bacteria and its relevance to chromate carcinogenesis. Nature. 1974 Aug 9;250(5466):493–495. doi: 10.1038/250493a0. [DOI] [PubMed] [Google Scholar]



