Abstract
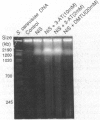
Some carcinogenic metal compounds [chromate(VI), Fe(III) nitrilotriacetate, cobalt(II), and nickel(II)[ induced formation of various oxygen radical species in the presence of hydrogen peroxide. These oxygen radicals were suggested to give different kinds of site-specific DNA damage; 8-hydroxyl-2'-deoxyguanosine formation is included in the DNA damage. Using pulsed-field gel electrophoresis, nickel sulfide was shown to induce oxidative DNA cleavage in cultured cells. On the basis of these findings, we have emphasized the role of oxygen radicals in metal carcinogenesis.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aiyar J., Berkovits H. J., Floyd R. A., Wetterhahn K. E. Reaction of chromium (VI) with hydrogen peroxide in the presence of glutathione: reactive intermediates and resulting DNA damage. Chem Res Toxicol. 1990 Nov-Dec;3(6):595–603. doi: 10.1021/tx00018a016. [DOI] [PubMed] [Google Scholar]
- Costa M., Mollenhauer H. H. Carcinogenic activity of particulate nickel compounds is proportional to their cellular uptake. Science. 1980 Jul 25;209(4455):515–517. doi: 10.1126/science.7394519. [DOI] [PubMed] [Google Scholar]
- Dizdaroglu M., Rao G., Halliwell B., Gajewski E. Damage to the DNA bases in mammalian chromatin by hydrogen peroxide in the presence of ferric and cupric ions. Arch Biochem Biophys. 1991 Mar;285(2):317–324. doi: 10.1016/0003-9861(91)90366-q. [DOI] [PubMed] [Google Scholar]
- Ebina Y., Okada S., Hamazaki S., Ogino F., Li J. L., Midorikawa O. Nephrotoxicity and renal cell carcinoma after use of iron- and aluminum-nitrilotriacetate complexes in rats. J Natl Cancer Inst. 1986 Jan;76(1):107–113. [PubMed] [Google Scholar]
- Inoue S., Kawanishi S. ESR evidence for superoxide, hydroxyl radicals and singlet oxygen produced from hydrogen peroxide and nickel(II) complex of glycylglycyl-L-histidine. Biochem Biophys Res Commun. 1989 Mar 15;159(2):445–451. doi: 10.1016/0006-291x(89)90012-0. [DOI] [PubMed] [Google Scholar]
- Inoue S., Kawanishi S. Hydroxyl radical production and human DNA damage induced by ferric nitrilotriacetate and hydrogen peroxide. Cancer Res. 1987 Dec 15;47(24 Pt 1):6522–6527. [PubMed] [Google Scholar]
- Ito K., Yamamoto K., Kawanishi S. Manganese-mediated oxidative damage of cellular and isolated DNA by isoniazid and related hydrazines: non-Fenton-type hydroxyl radical formation. Biochemistry. 1992 Nov 24;31(46):11606–11613. doi: 10.1021/bi00161a046. [DOI] [PubMed] [Google Scholar]
- Kasprzak K. S., Diwan B. A., Konishi N., Misra M., Rice J. M. Initiation by nickel acetate and promotion by sodium barbital of renal cortical epithelial tumors in male F344 rats. Carcinogenesis. 1990 Apr;11(4):647–652. doi: 10.1093/carcin/11.4.647. [DOI] [PubMed] [Google Scholar]
- Kawanishi S., Inoue S., Sano S. Mechanism of DNA cleavage induced by sodium chromate(VI) in the presence of hydrogen peroxide. J Biol Chem. 1986 May 5;261(13):5952–5958. [PubMed] [Google Scholar]
- Kawanishi S., Inoue S., Yamamoto K. Site-specific DNA damage induced by nickel(II) ion in the presence of hydrogen peroxide. Carcinogenesis. 1989 Dec;10(12):2231–2235. doi: 10.1093/carcin/10.12.2231. [DOI] [PubMed] [Google Scholar]
- Pott F., Ziem U., Reiffer F. J., Huth F., Ernst H., Mohr U. Carcinogenicity studies on fibres, metal compounds, and some other dusts in rats. Exp Pathol. 1987;32(3):129–152. doi: 10.1016/s0232-1513(87)80044-0. [DOI] [PubMed] [Google Scholar]
- Robison S. H., Cantoni O., Costa M. Strand breakage and decreased molecular weight of DNA induced by specific metal compounds. Carcinogenesis. 1982;3(6):657–662. doi: 10.1093/carcin/3.6.657. [DOI] [PubMed] [Google Scholar]
- Shi X. G., Sun X. L., Gannett P. M., Dalal N. S. Deferoxamine inhibition of Cr(V)-mediated radical generation and deoxyguanine hydroxylation: ESR and HPLC evidence. Arch Biochem Biophys. 1992 Mar;293(2):281–286. doi: 10.1016/0003-9861(92)90396-e. [DOI] [PubMed] [Google Scholar]
- Sugiyama M., Ando A., Ogura R. Vitamin B2-enhancement of sodium chromate (VI)--Induced DNA single strand breaks: ESR study of the action of vitamin B2. Biochem Biophys Res Commun. 1989 Mar 31;159(3):1080–1085. doi: 10.1016/0006-291x(89)92219-5. [DOI] [PubMed] [Google Scholar]
- Sugiyama M., Lin X. H., Costa M. Protective effect of vitamin E against chromosomal aberrations and mutation induced by sodium chromate in Chinese hamster V79 cells. Mutat Res. 1991 May;260(1):19–23. doi: 10.1016/0165-1218(91)90076-x. [DOI] [PubMed] [Google Scholar]
- Umemura T., Sai K., Takagi A., Hasegawa R., Kurokawa Y. Formation of 8-hydroxydeoxyguanosine (8-OH-dG) in rat kidney DNA after intraperitoneal administration of ferric nitrilotriacetate (Fe-NTA). Carcinogenesis. 1990 Feb;11(2):345–347. doi: 10.1093/carcin/11.2.345. [DOI] [PubMed] [Google Scholar]
- Yamamoto K., Inoue S., Yamazaki A., Yoshinaga T., Kawanishi S. Site-specific DNA damage induced by cobalt(II) ion and hydrogen peroxide: role of singlet oxygen. Chem Res Toxicol. 1989 Jul-Aug;2(4):234–239. doi: 10.1021/tx00010a004. [DOI] [PubMed] [Google Scholar]
- Yamamoto K., Kawanishi S. Site-specific DNA damage induced by hydrazine in the presence of manganese and copper ions. The role of hydroxyl radical and hydrogen atom. J Biol Chem. 1991 Jan 25;266(3):1509–1515. [PubMed] [Google Scholar]