Abstract
A method is described for high-throughput monitoring of DNA backbone integrity in plasmids and artificial chromosomes in solution. The method is based on the denaturation properties of double-stranded DNA in alkaline conditions and uses PicoGreen fluorochrome to monitor denaturation. In the present method, fluorescence enhancement of PicoGreen at pH 12.4 is normalised by its value at pH 8 to give a ratio that is proportional to the average backbone integrity of the DNA molecules in the sample. A good regression fit (r2 > 0.98) was obtained when results derived from the present method and those derived from agarose gel electrophoresis were compared. Spiking experiments indicated that the method is sensitive enough to detect a proportion of 6% (v/v) molecules with an average of less than two breaks per molecule. Under manual operation, validation parameters such as inter-assay and intra-assay variation gave values of <5% coefficient of variation. Automation of the method showed equivalence to the manual procedure with high reproducibility and low variability within wells. The method described requires as little as 0.5 ng of DNA per well and a 96-well microplate can be analysed in 12 min providing an attractive option for analysis of high molecular weight vectors. A preparation of a 116 kb bacterial artificial chromosome was subjected to chemical and shear degradation and DNA integrity was tested using the method. Good correlation was obtained between time of chemical degradation and shear rate with fluorescence response. Results obtained from pulsed- field electrophoresis of sheared samples were in agreement with those obtained using the microplate-based method.
INTRODUCTION
The increasing use of high molecular weight DNA vectors [bacterial artificial chromosomes (BACs), PACs, YACs, HACs] for gene expression and gene therapy protocols requires the development of sensitive analytical tools to monitor DNA quality and consistency during processing and storage (1–4). Additionally, since transfection efficiency of mammalian cells in culture has been shown to be dependent on DNA integrity (5–7), new methods to quickly assess the potency of DNA solutions are highly desirable.
Traditional methods for assessing the integrity of high molecular weight DNA include alkaline sucrose gradient centrifugation (8,9) and filter binding alkaline elution assays (10). Methods to detect apurinic sites usually involve 30 min of enzymatic digestion followed by agarose gel electrophoresis that may take from 0.5 to 24 h to run. Recently, two papers have been published that take advantage of the high sensitivity and specificity of the PicoGreen fluorochrome (11) for double-stranded DNA (dsDNA) to develop methods to measure DNA integrity (12,13). Levy et al. (12) reported a method based on the reversible denaturation properties of closed covalent circular molecules subjected to a heat (95°C) and rapid cool treatment. Since reversible denaturation conditions for vectors beyond 20 kb could prove to be cumbersome to establish, the use of this method is, in practical terms, limited to solutions containing low molecular weight vectors. Batel et al. (13) reported the use of PicoGreen under alkaline conditions (pH 12.4) to measure the rate of chromosomal DNA unwinding in cells and tissues that had been subjected to radiation and nitroquinoline treatment. Their assay, which is dependent on the number of cells per well, involves a 40 min lysis treatment in the presence of urea, SDS and EDTA followed by time-course evaluation of the extent of denaturation for <1 h.
In this paper, we present a method for detecting single-stranded DNA breaks in plasmids and BACs in solution that overcomes some of the limitations of previously described methods. The method, which takes advantage of the high stability of PicoGreen under alkaline conditions reported previously (13), is precise, rapid and results are independent of the amount of DNA in the well. Under controlled alkaline conditions, the degree of denaturation of dsDNA molecules is known to increase with increasing number of breaks and alkaline labile sites (i.e. depurinated sites). As unwinding begins independently at each break, the DNA remaining after the addition of alkali is proportional to the molecular weight of the dsDNA between these breaks. In the present method, fluorescence enhancement of PicoGreen at pH 12.4 is normalised by its value at pH 8 to give a ratio that is proportional to the average backbone integrity of the DNA molecules in the sample.
MATERIALS AND METHODS
Plasmid and bacterial cultures
Plasmid pSVβ (Promega Corp., Madison, WI), a 6.9 kb and pQR150 (14), a 20 kb plasmid, were transformed and propagated in Escherichia coli DH5α (Gibco-Life Technologies, Gaithersburg, MD). BAC-based episomal shuttle vector p5176, 116 kb in size (Wellcome Trust Centre for Human Genetics, University of Oxford, Oxford, UK) was transformed and propagated in E.coli DH10β (2). Culture, harvesting and storage conditions were as described in Levy et al. (12); cultures of E.coli DH5α pSVβ and DH5α pQR150 contained 100 µg ml–1 ampicillin and 50 µg ml–1 kanamycin, respectively. DH10β p5176 was grown with 12.5 µg ml–1 chloramphenicol on plates and 5 µg ml–1 chloramphenicol in shake flask cultures. Selective plates and nutrient media used for the preparation of p5176 were freshly prepared in light of the low concentration of antibiotic.
Plasmid purification
Pure DNA solutions of pSVβ, pQR150 and p5176 were obtained using Qiagen Maxi or Qiaprep Spin Miniprep kits and purification protocols for high and low copy number vectors (Qiagen Ltd, West Sussex, UK). DNA concentrations were determined by UV spectrophotometry at 260 nm.
Chemical and shear degradation
Pure preparations of pSVβ, pQR150 and p5176 were diluted to concentrations ranging from 100 to 900 ng ml–1 in TE buffer and incubated in a water bath at 60°C to accelerate the generation of DNA strand breaks through chemical degradation (15). The solutions (hereafter referred to as chemically degraded samples) were periodically sampled and aliquots kept at –20°C. Samples of 10 ml volume of pSVβ and p5176 at a concentration of 11 ng ml–1 in TE buffer were subjected to average shear rates between 1.6 × 104 s–1 and 3.3 × 105 s–1 using the methods described earlier (16,17).
Agarose gel electrophoresis
Plasmid pSVβ and pQR150 samples were loaded onto 0.8% agarose containing 0.05 µg ml–1 ethidium bromide and electrophoresed at 40–80 V, 220 mA for 2–5 h in Tris-borate electrophoresis buffer (9 mM Tris, 9 mM boric acid, 1 mM EDTA). BAC p5176 samples were loaded onto 1.0% agarose and electrophoresed at 6 V for 22.5 h on a Chef-DR® II pulsed-field electrophoresis system (Bio-Rad, Hercules, CA), initial switch time 0.8 s and final switch time 21.1 s. The gels were stained for 1 h after electrophoresis with ethidium bromide (0.05 µg ml–1 in Tris-borate buffer) and destained for 1 h before visualisation and analysis. The supercoiled (SC), open circular (OC) and linear DNA bands were analysed via densitometric scanning and SC DNA values were multiplied by a correction factor of 1.36 to account for the differential binding of ethidium bromide (18). Other minor bands, probably corresponding to chromosomal DNA contamination (19), were not taken into account for analysis. Gels were scanned using UVP 5000 Gel Documentation System and GelBase™ analysis software (Ultra Violet Products Ltd, Cambridge, UK).
Microplate-based fluorescence assay
In preliminary experiments two 50 µl aliquots of each sample were placed onto a microtitre plate. An equal volume of PicoGreen reagent (Molecular Probes, Leiden, The Netherlands) diluted 1:200 in TE buffer was added to each aliquot and incubated for 5 min. The fluorescence of samples was recorded using a Packard Fluorocount Microplate Fluorometer (Packard Instrument Company, Meriden, CT) at an excitation wavelength of 480 nm and emission of 520 nm giving a fluorescence value at pH 8. This was followed by addition of 100 µl of NaOH in the concentration range between 1 × 10–4 and 0.15 M to one aliquot of each sample to attain a final pH ranging from 9 to 13. The other aliquot was left as a control to standardise values with respect to pH 8. The identical results obtained between aliquots at pH 8 prompted us to use a single aliquot per sample in experiments reported in Figures 2–7. The NaOH solutions were freshly prepared before each experiment from a 10 M NaOH stock. To obtain pH 12.4 in the microwell a 0.1 M NaOH working solution was prepared and further diluted as required to obtain pH 12.6 ± 0.02, mixing equal volumes of this solution with TE pH 8 routinely gave a final pH of 12.4 ± 0.02. Under such conditions, a 5 and 10% volumetric increase or decrease of the NaOH working solution shifts the resulting experimental solution by 0.02 and 0.04 units, respectively. All measurements were corrected for background fluorescence from a blank containing equal volumes of TE buffer, NaOH solution and PicoGreen reagent.
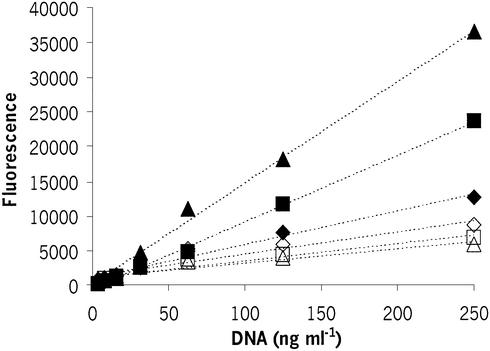
Figure 2.
Fluorescence enhancement of PicoGreen at pH 8 (closed symbols) and pH 12.4 (open symbols) as a function of DNA concentration for control pSVβ samples (closed diamonds, open diamonds) and samples chemically degraded by incubation at 60°C for 24 h (closed squares, open squares) and 44 h (closed triangles, open triangles). The dotted lines indicate regression fits (control DNA at pH 8, r2 = 0.996; 24 h degraded DNA at pH 8, r2 = 0.998; 44 h degraded DNA at pH 8, r2 = 0.986; control DNA at pH 12.4, r2 = 0.891; 24 h degraded DNA at pH 12.4, r2 = 0.904; 44 h degraded DNA at pH 12.4, r2 = 0.889).
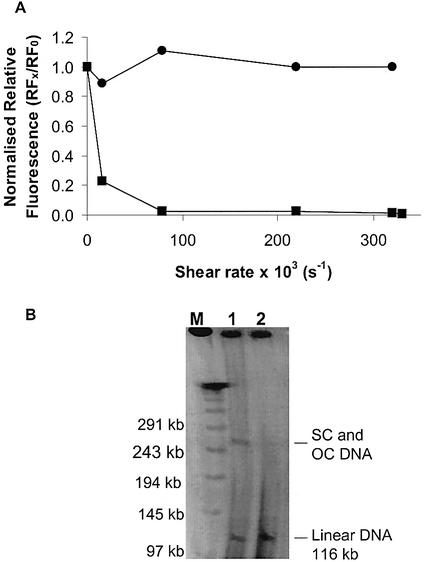
Figure 7.
(A) Effect of shear rate on RF for samples of pSVβ (circles) and p5176 (squares). RF values were normalised with respect to values at shear rate = 0. The DNA concentration was 11 ng ml–1. (B) Pulsed-field agarose gel electrophoresis of p5176 samples: (1) control and (2) sheared at 8 × 104 s–1 for 5 s. M, the λ multimer MW ladder.
Automation of the assay
A four-tip Packard Multiprobe II robotic workstation (Packard Instrument Company, Meridien, CT), WinPREP® software and integrated plate reader were used. DNA (60 µl) and PicoGreen (60 µl) were added to each well in single dispensation per aspiration mode, the 96-well plate was shaken for 5 s in the Fluorocount, incubated for 5 min and read. The plate was transferred to the working station where NaOH (120 µl) was added via multiple dispensation per aspiration mode, shaken for 5 s, incubated for 5 min and read.
RESULTS AND DISCUSSION
Assay development
The standard protocol for quantitation of dsDNA with PicoGreen (11) requires resuspension of the DNA sample and dilution of the fluorescent dye with TE at pH 8. Under such conditions, dsDNA molecules are expected to remain double stranded regardless of any single-stranded breaks or alkaline labile sites that they may contain. In contrast, at pH >8, denaturation (unwinding) begins independently at each break, the proportion of dsDNA remaining after a given period of exposure to alkali is considered to be proportional to the average molecular weight between breaks (20). A series of experiments was performed in order to evaluate whether the PicoGreen dye could be used for quantitation of dsDNA content in TE buffer at pH >8. Fluorescence enhancement of pSVβ control and chemically degraded samples at increasing pH (pH range 8–13) was measured (Fig. 1A). The fluorescence response decreased with increasing pH; the decrease being sharper for samples where single-stranded breaks had been intentionally generated by prolonged incubation at 60°C. Above pH 12.8, fluorescence values converged to a value just above background level. This was observed in all experiments and was thought to be due to the complete denaturation of the samples and/ or inactivation of the dye at such high pH. Under standard conditions (pH 8), for the same DNA concentration, the control sample gave a lower fluorescence signal than the chemically degraded samples. This is in agreement with previous findings that indicate that fluorescence enhancement of PicoGreen is dependent on DNA tertiary structure (11,12). To allow comparison with the control, fluorescence values obtained at pH >8 were normalised by dividing them by the respective values obtained at pH 8 and the data plotted as relative fluorescence (RF) against pH, as shown in Figure 1B. Different denaturation patterns were observed for the control and chemically degraded samples, the differences being greater between pH 9 and 12.4. The pH 12.4 was chosen for all subsequent experiments because of the robustness of response to minor variations in pH (Fig. 1B, inset).
Figure 1.
Effect of pH on (A) absolute and (B) RF enhancement of PicoGreen for control pSVβ samples (diamonds) and samples chemically degraded by incubation at 60°C for 24 h (squares) and 44 h (triangles). (B, inset) Magnification of (B) for the region between pH 12 and 12.8. RF was obtained by normalising fluorescence values at pH >8 with respect to fluorescence values obtained at pH 8. The plasmid concentration was 150 ng ml–1.
The relationship between fluorescence response at pH 12.4 and DNA concentration was assessed by serial dilution of control and degraded samples from 250 to 31 ng ml–1. The results shown in Figure 2 indicate direct proportionality between DNA concentration and fluorescence with correlation of r ≥ 0.89 at pH 12.4 for the three different samples tested. Using data of Figure 2, RF, the ratio of fluorescence at pH 12.4 and fluorescence at pH 8, was calculated. The results indicated that RF decreases with increasing average numbers of single-stranded breaks per DNA molecule in the sample and its value is independent of the amount of DNA in the well within the range studied.
The stability of the fluorescence response at pH 12.4 was evaluated with a time-course experiment for pSVβ control, 24 and 48 h chemically degraded samples. Following the addition of alkali, RF decreased sharply, converging to a steady value after 4 min, as shown in Figure 3. Statistical analysis showed that RF values taken between 4 and 7 min remained constant with a 5% coefficient of variation (CV), values measured between 3 and 11 min had a CV of 10%. Based on results shown in Figure 3, an incubation time of 5 min was chosen as an assay condition for subsequent experiments. Using a four-tipped multi-pipette operated manually, alkali may be added to a 96-well microplate in 2 min, using an eight-tipped pipette this procedure can take <1 min. An entire microplate can also be read in <1 min using the Fluorocount. Using a four-tipped automated liquid handling system, NaOH may be added to an entire 96-well microplate in 3 min, including tip washing and rinsing cycles.
Figure 3.
Effect of incubation time with PicoGreen at pH 12.4 on RF for control pSVβ samples (diamonds) and samples chemically degraded by incubation at 60°C for 24 h (squares) and 48 h (triangles). Dashed lines indicate 10% CV limits. The plasmid concentration was 320 ng ml–1. RF was obtained by normalising fluorescence values obtained at pH 12.4 by those obtained at pH 8.
Correlation with agarose gel electrophoresis
Samples of pSVβ subjected to increasing time (0–42 h) of chemical degradation were analysed with the microplate-based method and agarose gel electrophoresis. Densitometric scanning of the bands corresponding to the SC, OC and linear forms of the plasmid (Fig. 4A) was performed. Native samples (0 h) had a band corresponding to the SC form and fainter bands for OC and linear forms. For samples subjected to chemical degradation, the band corresponding to the SC form decreased with increasing incubation time with a concomitant increase of OC and linear forms. A correlation plot was constructed using data derived from the microplate method and densitometric scanning of the gel. As shown in Figure 4B, a good regression fit (r2 = 0.98) was obtained between RF and the ratio SC / (SC + OC + linear).
Figure 4.
(A) Agarose gel electrophoresis of pSVβ samples subjected to chemical degradation for 0–42 h. M, the DNA marker λ HindIII. (B) Correlation analysis between RF obtained from microplate-based fluorescence assay and percent SC plasmid obtained from densitometric scanning of SC, OC and linear DNA. The fluorescence results were the average of triplicate values, error bars represent the standard deviation. RF was obtained by normalising fluorescence values obtained at pH 12.4 by those obtained at pH 8. Solid line, linear regression of values, y = 0.605x + 0.302, r2 = 0.98. The DNA concentration in microplate-based fluorescence assay was 270 ng ml–1.
Assay sensitivity
Sensitivity of the microplate-based fluorescence assay was tested by evaluating the lowest percentage of molecules containing at least one strand break that could be reliably detected in a sample. Independent triplicate samples of control DNA spiked with increasing amounts of an OC DNA preparation were assayed and the results obtained are shown in Figure 5. The lowest percentage of spiked OC DNA at which the signal was statistically different from the control (Student’s t-test) was taken as the limit of sensitivity of the assay. Under the conditions used, this limit was found to be 6% where P < 0.05.
Figure 5.
Sensitivity analysis for microplate-based fluorescence assay. Control pSVβ samples were spiked with increasing amounts of an OC pSVβ preparation. OC DNA isoform was obtained by 24 h incubation at 60°C; the sample contained <12% linear DNA as assessed by densitometric scanning of an agarose gel. Results are the average of triplicates; error bars represent standard deviation.
Assay precision under manual and automated operation
Intra-assay precision under manual operation was determined by assaying three independent samples of pSVβ in triplicate; the %CV of RF obtained ranged from 2.9 to 4.1% (Table 1). In order to evaluate the precision of the assay under automated operation, eight replicates of three independent samples of pQR150 were tested; the %CV of results obtained were in all cases below 6% (Table 2). For the inter-assay precision, independent triplicates of three lots of pSVβ were assayed separately and compared; the %CV ranged from 1.8 to 3.5% (Table 3).
Table 1. Intra-assay precision for manual assay.
| Sample IDa | RF (values) | RF (mean ± SD) | CV (%) |
|---|---|---|---|
| A | 0.621 | 0.635 ± 0.026 | 4.1 |
| 0.620 | |||
| 0.665 | |||
| B | 0.705 | 0.716 ± 0.020 | 2.9 |
| 0.703 | |||
| 0.739 | |||
| C | 0.734 | 0.717 ± 0.029 | 4.0 |
| 0.734 | |||
| 0.684 |
aThree different samples of pSVβ were analysed in triplicate in the same 96-well microplate.
Table 2. Intra-assay precision for automated assay.
| Sample IDa | RF (values) | RF (mean ± SD) | CV (%) |
|---|---|---|---|
| A | 0.524, 0.491, 0.473 | 0.492 ± 0.025 | 5.1 |
| 0.528, 0.461, 0.484 | |||
| 0.503, 0.468 | |||
| B | 0.248, 0.232, 0.228 | 0.228 ± 0.012 | 5.4 |
| 0.238, 0.223, 0.234 | |||
| 0.212, 0.212 | |||
| C | 0.084, 0.088, 0.087 | 0.084 ± 0.003 | 3.5 |
| 0.080, 0.085, 0.084 | |||
| 0.080, 0.083 |
aThree different samples of control (A) and partially degraded pQR150 (B and C) were analysed in the same 96-well microplate.
Table 3. Inter-assay precision for manual assay.
| Sample IDa | RF (values) | RF (mean ± SD) | CV (%) |
|---|---|---|---|
| A | 0.699 | 0.713 ± 0.013 | 1.8 |
| 0.724 | |||
| 0.715 | |||
| B | 0.594 | 0.614 ± 0.022 | 3.5 |
| 0.636 | |||
| 0.611 | |||
| C | 0.538 | 0.549 ± 0.014 | 2.6 |
| 0.543 | |||
| 0.565 |
aThree independent assays for control (A) and partially degraded pSVβ samples (B and C) were performed.
Application of the assay to BAC DNA
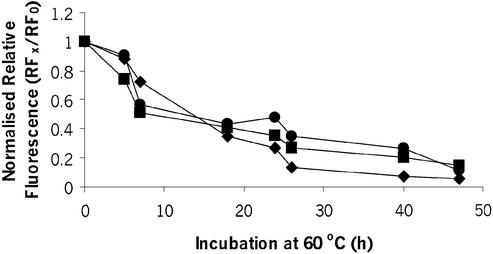
A preparation of BAC p5176 was incubated at 60°C to accelerate chemical degradation and samples were taken at regular intervals and assayed as described in the previous section. Preparations of pSVβ and pQR150 were subjected to the same treatment and assayed in parallel as controls. For the BAC DNA and the two plasmids tested, normalised RF values (RFx / RF0) decreased sharply with time between 0 and 10 h, but decreased at a slower rate between 10 and 48 h (Fig. 6).
Figure 6.
Effect of duration of chemical degradation through incubation at 60°C on RF for pSVβ (circles), pQR150 (diamonds) and p5176 (squares). RF values were normalised with respect to values at time = 0. The DNA concentration of pSVβ and p5176 was 300 ng ml–1, and the DNA concentration of pQR150 was 890 ng ml–1.
Samples of p5176 were subjected to shear at average rates of 1.6 × 104–3.3 × 105 s–1 using equipment and methods described previously (16,17). A sample of pSVβ was treated in parallel as a control. The normalised RF for p5176 (116 kb) decreased sharply at relatively low shear rates (∼1 × 103 s–1), indicating that considerable damage to DNA backbone had occurred (Fig. 7A). As expected, pSVβ (6.9 kb) showed no decrease in RF values over the shear rates tested (Fig. 7A). To confirm the results obtained with p5176, a pulsed-field gel electrophoresis (21) was carried out using control and sheared preparations. The control DNA showed bands corresponding to circular (SC and OC) and linear forms (Fig. 7B). The sample subjected to a shear rate of 8 ×104 s–1 (lane 2) had no circular forms remaining and only linear forms were visualised in the gel. These results are in agreement with results obtained using the microplate-based assay and confirm that RF values can be used as a good indicator of the average backbone integrity of high molecular weight vectors.
CONCLUSION
In the present paper, we describe a microplate-based method that provides rapid information on the backbone integrity of dsDNA molecules in solution. The method is simple, takes 12 min per 96-well microplate and requires small amounts of sample. The method is amenable to high-throughput automation using a robotic liquid handling system integrated with a microplate fluorometer. The method was used successfully to rapidly evaluate chemical and shear-induced damage of an artificial chromosome preparation (Figs 6 and 7). The method provides an attractive option for analysis of backbone integrity of high molecular weight vectors that would otherwise require more time-consuming and labour-intensive methods. Further more, the method has the potential to detect alkaline labile sites, such as apurinic sites, that may not be detected using electrophoretic methods.
Acknowledgments
ACKNOWLEDGEMENTS
The authors would like to thank Dr John Ward (Department of Biochemistry and Molecular Biology, UCL) for advice and critical reading of the manuscript, Dr Michael James and Dr Richard Wade-Martins for providing p5176 and Simyee Kong for her help with the automation of the assay. C.R. is a recipient of a UCL Overseas Research Studentship, a Denys Holland Scholarship and a Harold Hyam Wingate Foundation award. UCL Innovative Manufacturing Research Centre (IMRC) for Bioprocessing is sponsored by EPSRC. The council support is gratefully acknowledged.
REFERENCES
- 1.Butler V.A. (1996) Points to Consider on Plasmid DNA Vaccines for Preventative Infectious Disease Indications. Office of Vaccine Research and Review, Centre for Biologics Evaluation and Research, Food and Drug Administration.
- 2.Wade-Martins R., Frampton,J. and James,M.R. (1999) Long-term stability of large insert genomic DNA episomal shuttle vectors in human cells. Nucleic Acids Res., 27, 1674–1682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Campeau P., Chapdelaine,P., Seigneurin-Venin,S., Massie,B. and Tremblay,J.P. (2001) Transfection of large plasmids in primary human myoblasts. Gene Ther., 8, 1387–1394. [DOI] [PubMed] [Google Scholar]
- 4.Compton S.T., Henning,K.A., Chen,M., Mansoura,M.K. and Ashlock,M.A. (1999) An improved method for routine preparation of intact artificial chromosome DNA (340–1000 kb) for transfection into human cells. Nucleic Acids Res., 27, 1762–1765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cherng J.Y., Schuurmans-Nieuwenbroek,E., Jiskoot,W., Talsma,H., Zuidam,N.J., Hennink,E. and Crommelin,D.J.A. (1999) Effect of DNA topology on the transfection efficiency of poly ((2-dimethylamino)ethyl methacrylate)-plasmid complexes. J. Control Release, 60, 343–353. [DOI] [PubMed] [Google Scholar]
- 6.Xie T.D., Sun,L., Zhao,J.A., Fuchs,T.Y. and Tsong,T.Y. (1992) Study of mechanisms of electric field-induced DNA transfection. IV. Effect of DNA topology on cell uptake and transfection efficiency. Biophys. J., 63, 1026–1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Weintraub H., Cheng,P.F. and Conrad,K. (1986) Expression of transfected DNA depends on DNA topology. Cell, 46, 115–122. [DOI] [PubMed] [Google Scholar]
- 8.Bermudez E., Ferng,S.-F., Castro,C.E. and Mustafa,M.G. (1999) DNA strand breaks caused by exposure to ozone and nitrogen dioxide. Environ. Res., 81, 72–80. [DOI] [PubMed] [Google Scholar]
- 9.Brambilla G., Martelli,A., Robbiano,L., Carrozzino,R., Port Puglia,C., Mattioli,F. and Angiola,M. (2000) DNA damage induced by 3,3-dimethoxybenzidine in liver and urinary bladder cells of rats and humans. Toxicol. Sci., 53, 71–76. [DOI] [PubMed] [Google Scholar]
- 10.Guan J., DiBiase,S. and Iliakis,G. (2000) The catalytic subunit DNA-dependent protein kinase (DNA-PKcs) facilitates recovery from radiation-induced inhibition of DNA replication. Nucleic Acids Res., 28, 1183–1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Singer V.L., Jones,L.J., Yue,S.T. and Haughland,R.P. (1997) Characterisation of PicoGreen reagent and development of a fluorescent based solution assay for double-stranded DNA quantitation. Anal. Biochem., 249, 228–238. [DOI] [PubMed] [Google Scholar]
- 12.Levy M.S., Lotfian,P., O’Kennedy,R., Lo-Yim,M.Y. and Ayazi Shamlou,P. (2000) Quantitation of supercoiled circular content in plasmid DNA solutions using a fluorescence based method. Nucleic Acids Res., 28, e57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Batel R., Jaksic,Z., Bihari,N., Hamer,B., Fafandel,M., Chauvin,C., Schrder,H.C., Muller,W.E.G. and Zahn,R. (1999) A microplate assay for DNA damage determination (fast micromethod) in cell suspensions and solid tissues. Anal. Biochem., 270, 195–200. [DOI] [PubMed] [Google Scholar]
- 14.Jackson G., Sherestha,S. and Ward,J.M. (1995) Metabolic engineering of the toluene degradation pathway. J. Cell. Biochem., S21A CIA, 48. [Google Scholar]
- 15.Evans R.K., Xu,Z., Bohannen,K.E., Wang,B., Bruner,M.W. and Volkin,D.B. (1999) Evaluation of degradation pathways for plasmid DNA in pharmaceutical formulations via accelerated stability studies. J. Pharm. Sci., 89, 76–87. [DOI] [PubMed] [Google Scholar]
- 16.Levy M.S., Collins,I.J., Yim,S.S., Ward,J.M., Titchener-Hooker,N., Ayazi Shamlou,P. and Dunnill,P. (1999) Effect of shear on plasmid DNA in solution. Bioprocess Eng., 20, 7–13. [Google Scholar]
- 17.Levy M.S., O’Kennedy,R.D., Shamlou,P.A. and Dunnill,P. (2000) Biochemical engineering approaches to the challenges of producing pure plasmid DNA. Trends Biotechnol., 18, 296–305. [DOI] [PubMed] [Google Scholar]
- 18.Projan S.J., Carelton,S. and Novick,R.P. (1983) Determination of plasmid copy number by fluorescence densitometry. Plasmid, 9, 182–190. [DOI] [PubMed] [Google Scholar]
- 19.Levy M.S., Collins,I.J., Ward,J.M., Ayazi Shamlou,P. and Dunnill,P. (2000) Removal of contaminant nucleic acids by nitrocellulose filtration during pharmaceutical-grade plasmid DNA processing. J. Biotechnol., 76, 197–205. [DOI] [PubMed] [Google Scholar]
- 20.Rydberg B. (1975) The rate of strand separation in alkali of DNA of irradiated mammalian cells. Radiat. Res., 61, 274–287. [PubMed] [Google Scholar]
- 21.Akerman B. and Cole,K.D. (2002) Electrophoretic capture of circular DNA in gels. Electrophoresis, 23, 2549–2561. [DOI] [PubMed] [Google Scholar]