Abstract
The potential role of iron and copper and the involvement of hydroxyl radicals in the DNA cleavage caused by chromate and glutathione (GSH) has been investigated. We have also studied the ability of chromate, on reaction with ascorbate as well as in mixed solutions of ascorbate and GSH, to cause DNA strand breaks. In both fully demetalated and conventional (i.e., metal contaminated) systems, chromate and GSH induced similar numbers of DNA strand breaks. This observation suggests that traces of iron or copper contaminating the reaction mixtures do not play a major role in the DNA cleavage caused by chromate and GSH. A series of hydroxyl radical scavengers exhibited a protective influence on the induction of DNA strand breaks. However, glucose and sucrose, both strong hydroxyl radical scavengers, showed no concentration-dependent inhibition of DNA cleavage. Competition kinetics studies yielded apparent rate constants that were not consistent with hydroxyl radicals being the species responsible for DNA strand breaks. Ascorbate in combination with chromate was also found to induce strand breaks in DNA; this damage could be attributed to reactive intermediates generated during the reduction. When mixed systems of ascorbate and GSH in the presence of chromate were investigated, there were clearly interactions between the two reductants.
Full text
PDF
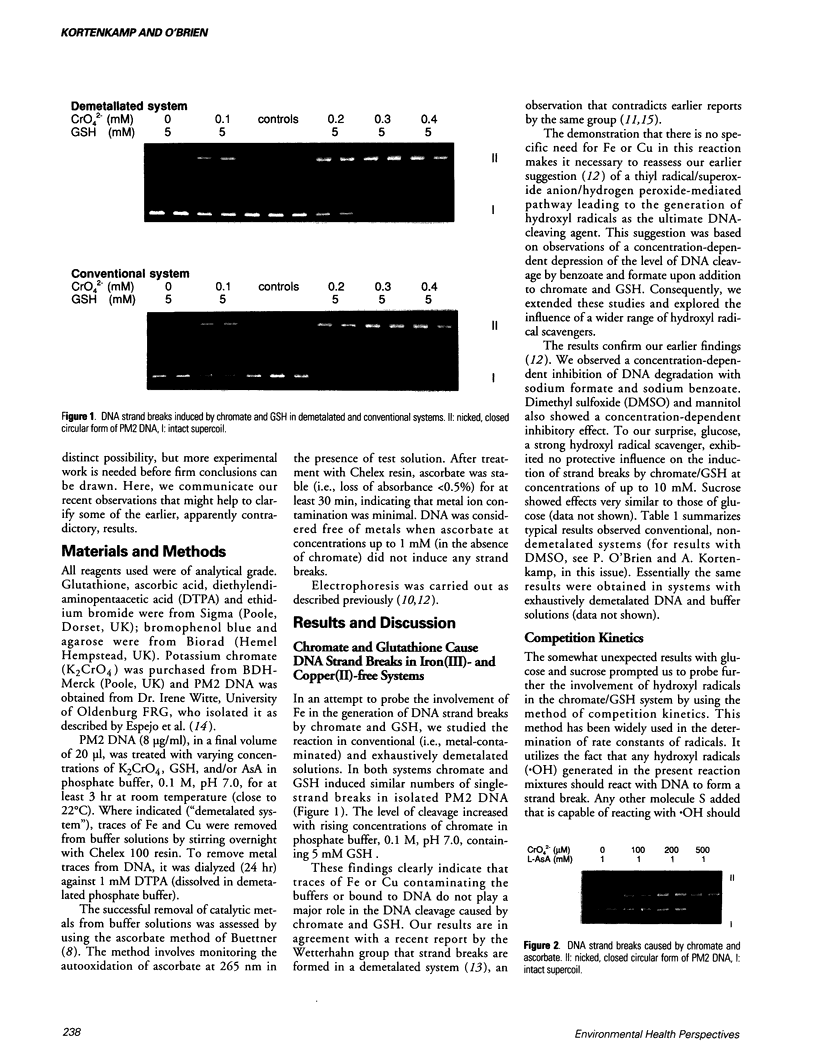

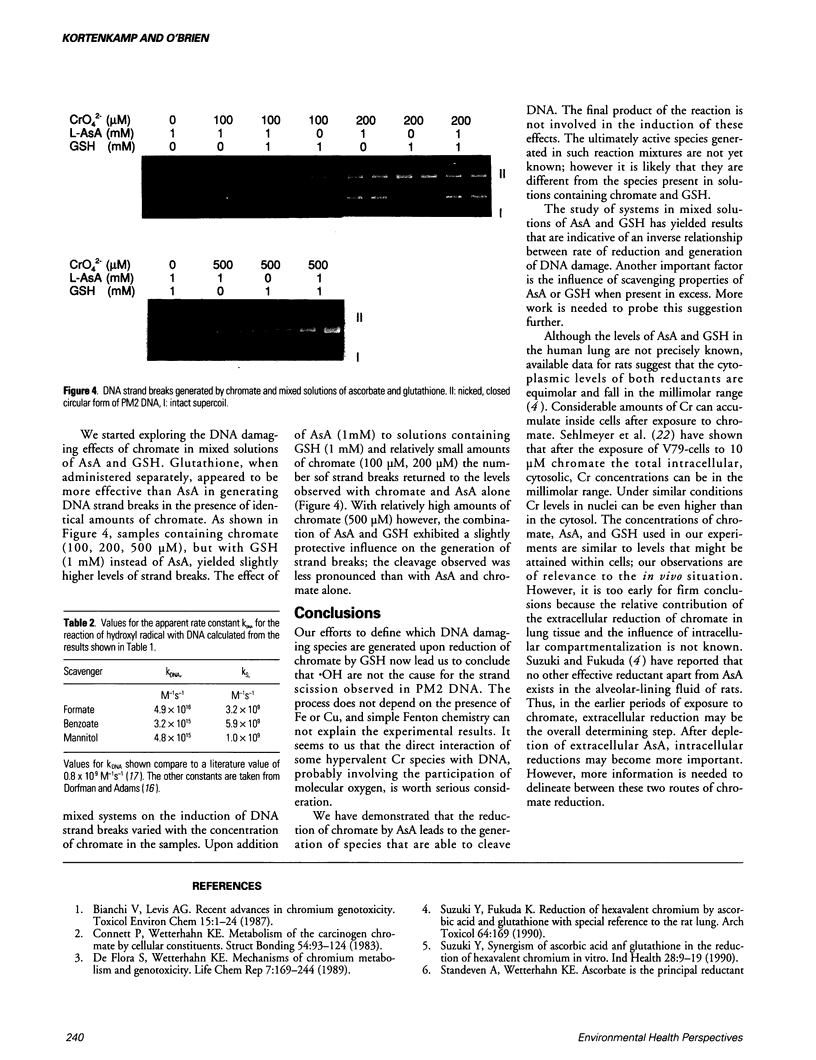

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Borges K. M., Boswell J. S., Liebross R. H., Wetterhahn K. E. Activation of chromium(VI) by thiols results in chromium(V) formation, chromium binding to DNA and altered DNA conformation. Carcinogenesis. 1991 Apr;12(4):551–561. doi: 10.1093/carcin/12.4.551. [DOI] [PubMed] [Google Scholar]
- Borges K. M., Wetterhahn K. E. Chromium bound to DNA alters cleavage by restriction endonucleases. Chem Res Toxicol. 1991 Nov-Dec;4(6):638–641. doi: 10.1021/tx00024a007. [DOI] [PubMed] [Google Scholar]
- Buettner G. R. In the absence of catalytic metals ascorbate does not autoxidize at pH 7: ascorbate as a test for catalytic metals. J Biochem Biophys Methods. 1988 May;16(1):27–40. doi: 10.1016/0165-022x(88)90100-5. [DOI] [PubMed] [Google Scholar]
- Espejo R. T., Canelo E. S., Sinsheimer R. L. DNA of bacteriophage PM2: a closed circular double-stranded molecule. Proc Natl Acad Sci U S A. 1969 Aug;63(4):1164–1168. doi: 10.1073/pnas.63.4.1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrell R. P., Judd R. J., Lay P. A., Dixon N. E., Baker R. S., Bonin A. M. Chromium(V)-induced cleavage of DNA: are chromium(V) complexes the active carcinogens in chromium(VI)-induced cancers? Chem Res Toxicol. 1989 Jul-Aug;2(4):227–229. doi: 10.1021/tx00010a002. [DOI] [PubMed] [Google Scholar]
- Kortenkamp A., Oetken G., Beyersmann D. The DNA cleavage induced by a chromium(V) complex and by chromate and glutathione is mediated by activated oxygen species. Mutat Res. 1990 Oct;232(2):155–161. doi: 10.1016/0027-5107(90)90120-s. [DOI] [PubMed] [Google Scholar]
- Kortenkamp A., Ozolins Z., Beyersmann D., O'Brien P. Generation of PM2 DNA breaks in the course of reduction of chromium(VI) by glutathione. Mutat Res. 1989 Feb;216(1):19–26. doi: 10.1016/0165-1161(89)90019-8. [DOI] [PubMed] [Google Scholar]
- Sehlmeyer U., Hechtenberg S., Klyszcz H., Beyersmann D. Accumulation of chromium in Chinese hamster V79-cells and nuclei. Arch Toxicol. 1990;64(6):506–508. doi: 10.1007/BF01977636. [DOI] [PubMed] [Google Scholar]
- Standeven A. M., Wetterhahn K. E. Ascorbate is the principal reductant of chromium (VI) in rat liver and kidney ultrafiltrates. Carcinogenesis. 1991 Sep;12(9):1733–1737. doi: 10.1093/carcin/12.9.1733. [DOI] [PubMed] [Google Scholar]
- Standeven A. M., Wetterhahn K. E. Is there a role for reactive oxygen species in the mechanism of chromium(VI) carcinogenesis? Chem Res Toxicol. 1991 Nov-Dec;4(6):616–625. doi: 10.1021/tx00024a003. [DOI] [PubMed] [Google Scholar]
- Suzuki Y., Fukuda K. Reduction of hexavalent chromium by ascorbic acid and glutathione with special reference to the rat lung. Arch Toxicol. 1990;64(3):169–176. doi: 10.1007/BF02010721. [DOI] [PubMed] [Google Scholar]
- Suzuki Y. Synergism of ascorbic acid and glutathione in the reduction of hexavalent chromium in vitro. Ind Health. 1990;28(1):9–19. doi: 10.2486/indhealth.28.9. [DOI] [PubMed] [Google Scholar]






