Abstract
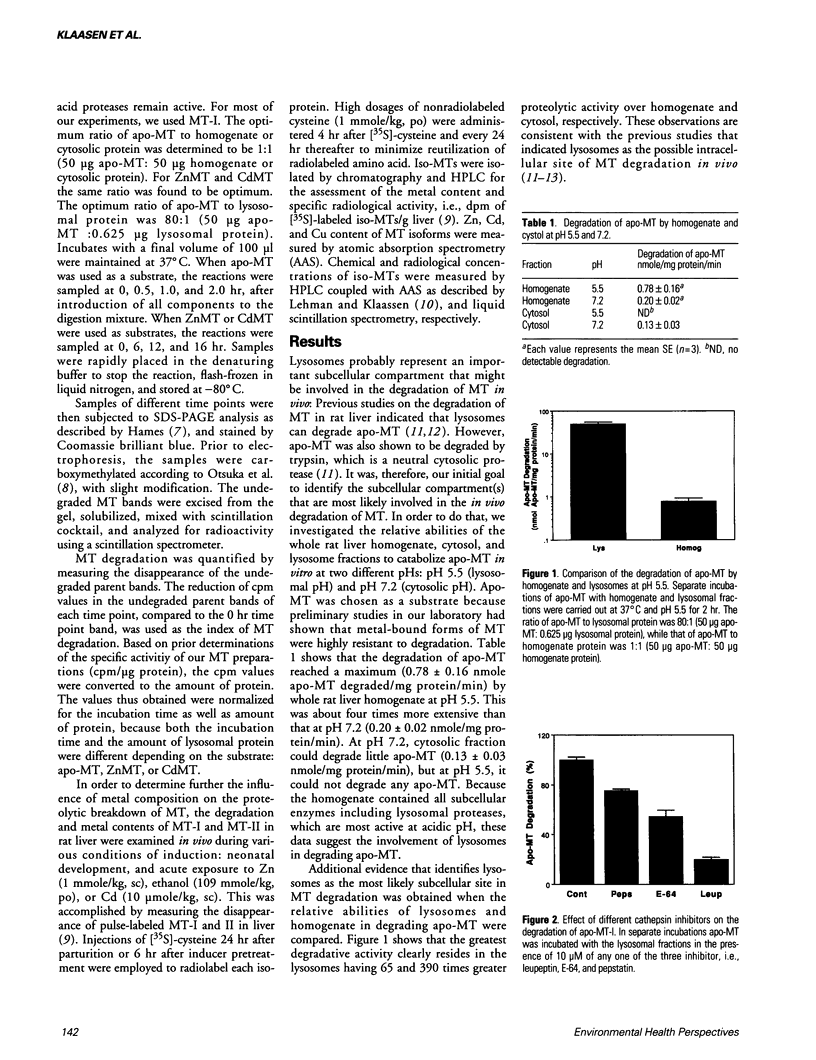
Degradation of metallothionein (MT) from rat liver was examined. Degradation of apo-MT by liver homogenate was greater than that by cytosol. At pH 5.5, degradation by homogenate was more than that at pH 7.2. These findings suggest that proteases that function at acidic pH are probably involved in MT degradation. Because lysosomes are the principal subcellular organelles that contain acid proteases (cathepsins), we compared the degradation of apo-MT by lysosomes and cytosol. Apo-MT was degraded about 400 times faster by lysosomal fraction than by cytosolic fraction. To determine the relative importance of different cathepsins, we used different inhibitors. Leupeptin, which inhibits cathepsins B and L, inhibited the degradation of apo-MT by 80%, implying that cathepsins B and/or L might be very important in the intracellular turnover of MT. Cathepsin D appeared to be the least significant, because apo-MT degradation was reduced by about 20% by inhibiting cathepsin D. When we extended this study with purified cathepsins, we obtained the same answer, i.e., the ability of different cathepsins to degrade apo-MT was in the following order: cathepsin B >> cathepsin C > cathepsin D. While apo-MT was susceptible to degradation, ZnMT and CdMT were highly resistant to degradation. Coincubation of ZnMT or CdMT with either lysosomal extract or purified cathepsins did not result in any appreciable degradation even after 16 hr. However, longer incubations did result in some degradation, especially by purified cathepsin B. Interestingly, CdMT degraded little faster than ZnMT by both lysosomal extract as well as purified cathepsin B.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chen R. W., Whanger P. D., Weswig P. H. Biological function of metallothionein. I. Synthesis and degradation of rat liver metallothionein. Biochem Med. 1975 Feb;12(2):95–105. doi: 10.1016/0006-2944(75)90100-3. [DOI] [PubMed] [Google Scholar]
- Choudhuri S., McKim J. M., Jr, Klaassen C. D. Role of hepatic lysosomes in the degradation of metallothionein. Toxicol Appl Pharmacol. 1992 Jul;115(1):64–71. doi: 10.1016/0041-008x(92)90368-3. [DOI] [PubMed] [Google Scholar]
- Clough S. R., Mitra R. S., Kulkarni A. P. Qualitative and quantitative aspects of human fetal liver metallothioneins. Biol Neonate. 1986;49(5):241–254. doi: 10.1159/000242538. [DOI] [PubMed] [Google Scholar]
- Feldman S. L., Cousins R. J. Degradation of hepatic zinc-thionein after parenteral zinc administration. Biochem J. 1976 Dec 15;160(3):583–588. doi: 10.1042/bj1600583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman S. L., Failla M. L., Cousins R. J. Degradation of rat liver metallothioneins in vitro. Biochim Biophys Acta. 1978 Dec 18;544(3):638–646. doi: 10.1016/0304-4165(78)90338-0. [DOI] [PubMed] [Google Scholar]
- Funabiki R., Yagasaki K., Shirakawa C., Sugita H., Ishiura S. Activity measurement of lysosomal cysteine proteinases, cathepsins B, H and L, in crude tissue extracts, and their relation to the fractional rate of protein degradation. Int J Biochem. 1990;22(11):1303–1306. doi: 10.1016/0020-711x(90)90313-r. [DOI] [PubMed] [Google Scholar]
- Hamer D. H. Metallothionein. Annu Rev Biochem. 1986;55:913–951. doi: 10.1146/annurev.bi.55.070186.004405. [DOI] [PubMed] [Google Scholar]
- Ii K., Hizawa K., Kominami E., Bando Y., Katunuma N. Different immunolocalizations of cathepsins B, H, and L in the liver. J Histochem Cytochem. 1985 Nov;33(11):1173–1175. doi: 10.1177/33.11.4056381. [DOI] [PubMed] [Google Scholar]
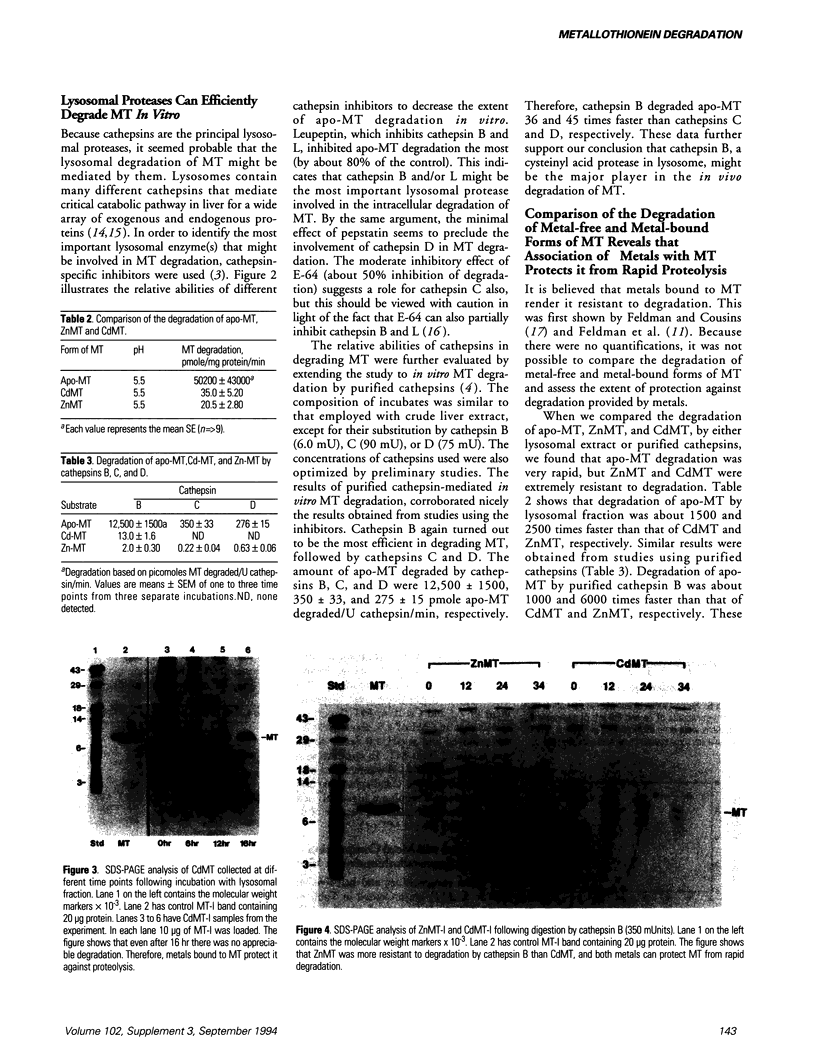
- Kershaw W. C., Klaassen C. D. Degradation and metal composition of hepatic isometallothioneins in rats. Toxicol Appl Pharmacol. 1992 Jan;112(1):24–31. doi: 10.1016/0041-008x(92)90275-w. [DOI] [PubMed] [Google Scholar]
- Lehman L. D., Klaassen C. D. Separation and quantitation of metallothioneins by high-performance liquid chromatography coupled with atomic absorption spectrophotometry. Anal Biochem. 1986 Mar;153(2):305–314. doi: 10.1016/0003-2697(86)90097-7. [DOI] [PubMed] [Google Scholar]
- McKim J. M., Jr, Choudhuri S., Klaassen C. D. In vitro degradation of apo-, zinc-, and cadmium-metallothionein by cathepsins B, C, and D. Toxicol Appl Pharmacol. 1992 Sep;116(1):117–124. doi: 10.1016/0041-008x(92)90152-i. [DOI] [PubMed] [Google Scholar]
- Nartey N. O., Banerjee D., Cherian M. G. Immunohistochemical localization of metallothionein in cell nucleus and cytoplasm of fetal human liver and kidney and its changes during development. Pathology. 1987 Jul;19(3):233–238. doi: 10.3109/00313028709066555. [DOI] [PubMed] [Google Scholar]
- Nettesheim D. G., Engeseth H. R., Otvos J. D. Products of metal exchange reactions of metallothionein. Biochemistry. 1985 Nov 19;24(24):6744–6751. doi: 10.1021/bi00345a003. [DOI] [PubMed] [Google Scholar]
- Osada J., Aylagas H., Sánchez-Prieto J., Sánchez-Vegazo I., Palacios-Alaiz E. Isolation of rat liver lysosomes by a single two-phase partition on dextran/polyethylene glycol. Anal Biochem. 1990 Mar;185(2):249–253. doi: 10.1016/0003-2697(90)90288-k. [DOI] [PubMed] [Google Scholar]
- Otsuka F., Koizumi S., Kimura M., Ohsawa M. Silver staining for carboxymethylated metallothioneins in polyacrylamide gels. Anal Biochem. 1988 Jan;168(1):184–192. doi: 10.1016/0003-2697(88)90027-9. [DOI] [PubMed] [Google Scholar]
- Sendelbach L. E., Klaassen C. D. Kidney synthesizes less metallothionein than liver in response to cadmium chloride and cadmium-metallothionein. Toxicol Appl Pharmacol. 1988 Jan;92(1):95–102. doi: 10.1016/0041-008x(88)90231-1. [DOI] [PubMed] [Google Scholar]
- Soumillion A., Van Damme J., De Ley M. Cloning and specific polymerised-chain-reaction amplification of a third charge-separable human metallothionein isoform. Eur J Biochem. 1992 Nov 1;209(3):999–1004. doi: 10.1111/j.1432-1033.1992.tb17374.x. [DOI] [PubMed] [Google Scholar]
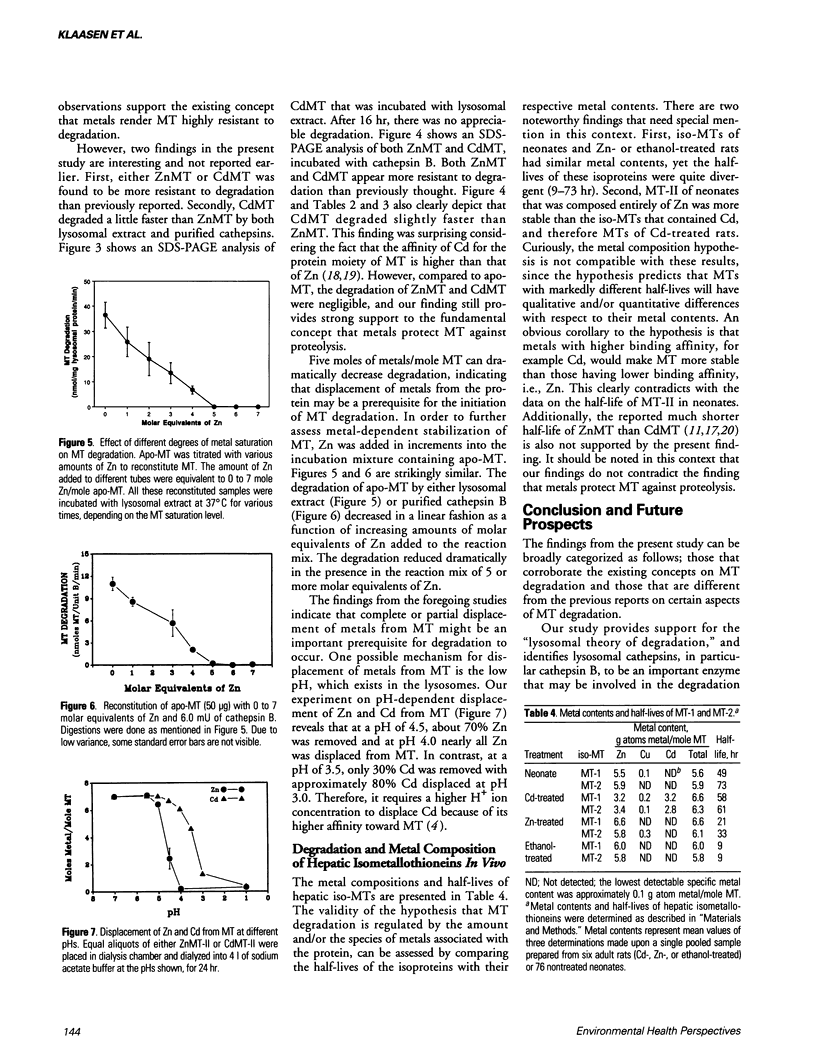
- Steinebach O. M., Wolterbeek B. T. Metallothionein biodegradation in rat hepatoma cells: a compartmental analysis aided 35S-radiotracer study. Biochim Biophys Acta. 1992 Apr 22;1116(2):155–165. doi: 10.1016/0304-4165(92)90112-8. [DOI] [PubMed] [Google Scholar]
- Tsujikawa K., Imai T., Kakutani M., Kayamori Y., Mimura T., Otaki N., Kimura M., Fukuyama R., Shimizu N. Localization of metallothionein in nuclei of growing primary cultured adult rat hepatocytes. FEBS Lett. 1991 Jun 3;283(2):239–242. doi: 10.1016/0014-5793(91)80597-v. [DOI] [PubMed] [Google Scholar]
- Wong K. L., Klaassen C. D. Isolation and characterization of metallothionein which is highly concentrated in newborn rat liver. J Biol Chem. 1979 Dec 25;254(24):12399–12403. [PubMed] [Google Scholar]