Abstract
Condensin and cohesin are chromosomal protein complexes required for chromosome condensation and sister chromatid cohesion, respectively. They commonly contain the SMC (structural maintenance of chromosomes) subunits consisting of a long coiled-coil with the terminal globular domains and the central hinge. Condensin and cohesin holo-complexes contain three and two non-SMC subunits, respectively. In this study, DNA interaction with cohesin and condensin complexes purified from fission yeast was investigated. The DNA reannealing activity is strong for condensin SMC heterodimer but weak for holo-condensin, whereas no annealing activity is found for cohesin heterodimer SMC and Rad21-bound heterotrimer complexes. One set of globular domains of the same condensin SMC is essential for the DNA reannealing activity. In addition, the coiled-coil and hinge region of another SMC are needed. Atomic force microscopy discloses the molecular events of DNA reannealing. SMC assembly that occurs on reannealing DNA seems to be a necessary intermediary step. SMC is eliminated from the completed double-stranded DNA. The ability of heterodimeric SMC to reanneal DNA may be regulated in vivo possibly through the non-SMC heterotrimeric complex.
Keywords: chromosome/cohesion/condensation/fission yeast/SMC
Introduction
Condensin and cohesin are the evolutionarily conserved protein complexes essential for chromosome condensation in mitosis and sister chromatid cohesion after replication, respectively (Hirano, 2000; Nasmyth, 2001). They execute important functions in maintaining the fidelity of chromosome inheritance from mother to daughter cells. The complexes contain subunits called SMCs (structural maintenance of chromosomes), which have a long coiled-coil with a central hinge region and N- and C-terminal ATP-interacting globular domains (Strunnikov and Jessberger, 1999; Cobbe and Heck, 2000; Hirano, 2002). In eukaryotes, SMC proteins exist as heterodimers bound by additional non-SMC subunits.
Condensin consists of five distinct subunits, two SMCs and three non-SMCs forming the 13S complex (Hirano et al., 1997; Sutani et al., 1999; Freeman et al., 2000). In the fission yeast Schizosaccharomyces pombe, temperature-sensitive mutants in cut3 and cut14 defective in the SMC subunit chromosomes fail to condense during mitosis (Saka et al., 1994). Similar defective phenotypes have been identified in budding yeast Saccharomyces cerevisiae, Drosophila and Caenorhabditis elegans (Strunnikov et al., 1995; Freeman et al., 2000; Steffensen et al., 2001; Hagstrom et al., 2002). Cut3 and Cut14 are similar to Smc4 and Smc2, respectively. Fission yeast cnd2 defective in one non-SMC subunit fails in DNA repair and activation of the checkpoint kinase Cds1/Chk2 as well as in condensation, indicating that condensin has interphase as well as mitotic functions (Aono et al., 2002). Cohesin also has two SMC subunits different from those of condensin and contains additional non-SMC subunits (Losada et al., 1998; Toth et al., 1999; Tomonaga et al., 2000). In S.pombe, two cohesin SMC subunits, Psm1 and Psm3 (similar to Smc1 and Smc3, respectively), co-precipitate with Rad21, a homologue of Scc1, but only weakly with Psc3 (a Scc3 homologue, Tomonaga et al., 2000). One non-SMC subunit (Scc1/Rad21) is cleaved by a separase in anaphase, a step leading to sister chromatid separation (Uhlmann et al., 1999, 2000; Tomonaga et al., 2000).
What are the molecular activities of the complexes? Hirano and associates have demonstrated that the frog condensin complex exhibits ATP-dependent DNA supercoiling activity which is essential for condensation in vitro (Kimura and Hirano, 1997; Kimura et al., 1999). The activity is stoichiometric rather than catalytic (Kimura and Hirano, 1997). On the other hand, cohesin complex purified from HeLa cell extracts promoted intermolecular catenation of circular DNA in the presence of topoisomerase II (Losada and Hirano, 2001).
There may exist two principal approaches to understand the molecular functions of condensin and cohesin. One is to search for and identify the activity of intact whole complexes, and then to integrate the knowledge about the complexes with cell cycle and mitotic regulation. Hirano’s group showed that interphase 13S condensin is inactive and that active Cdc2 kinase is necessary to activate the positive supercoiling activity (Kimura et al., 1998). The other approach would be to divide the complexes into individual subunits or subcomplexes, and attempt to identify any activities that they have, though they may represent only partial activity of the whole complex. The stable, two-member SMC subcomplex of condensin previously was shown to promote DNA reannealing from complementary single-stranded (ss) DNA to double-stranded (ds) DNA (Sutani and Yanagida, 1997). The activity was not detected for the SMC complex containing a mutant subunit derived from the temperature-sensitive cut14 strain, which was defective in chromosome condensation. The activity of promoting ssDNA reannealing in vitro has been also found in the mammalian recombination complex RC-1 (Jessberger et al., 1996) and in the Bacillus subtilis SMC homodimeric complex (Hirano and Hirano, 1998).
In this study, we purified SMC heterodimer, non-SMC heterotrimer and heteropentameric holo-condensin, and also cohesin SMC heterodimer and Rad21-containing heterotrimeric complexes from fission yeast, and examined their DNA-binding abilities and reannealing from complementary ssDNAs. The DNA-binding behaviour of the heterodimeric SMC of cohesin and condensin was strikingly different. We wanted to understand the molecular basis for these differences and thus made a number of truncated SMCs. To gain information about the mechanism of DNA reannealing by condensin SMC, the time course events were investigated using atomic force microscopy (AFM). AFM is a convenient tool to observe condensin and cohesin (Yoshimura et al., 2002) as the procedures to treat specimens for staining and vacuum drying are not necessary. Intermediate protein–DNA assembled structures were found in abundance before the completion of DNA reannealing. Protein–protein interaction is a feature of condensin SMC–DNA interaction.
Results
Characterization of purified cohesin heterodimer SMC
To isolate the cohesin heterodimeric SMC complex of S.pombe, the genes for Psm1 and Psm3 were tagged with 8Myc and 3haemagglutinin (HA)-His6, respectively. The tagged genes were functional as they could replace the wild-type genes (data not shown). Plasmids carrying the tagged genes under the inducible promoter nmt1 (REP1; Maundrell, 1990) were introduced into S.pombe. Psm1-8Myc and Psm3-3HA-His6 were thus overexpressed simultaneously in the absence of thiamine (the promoter, on). The culture conditions and purification procedures for the Psm1–Psm3 complex are described in Materials and methods. The clarified supernatant was loaded onto an Ni-NTA column (Figure 1A). Eluate patterns stained with Coomassie Brilliant Blue (CBB) showed the co-elution of Psm1-8Myc and Psm3-3HA-His6 (relative mol wts 151 and 135 kDa, respectively). Their identification was confirmed by immunoblot using antibodies against Myc and HA, respectively (right panel). The purity of the heterodimer was ∼80%.
Fig. 1. Purification of cohesin SMC heterodimer. (A) Extracts of an S.pombe strain overexpressing Psm1-8Myc and Psm3-3HA-His6 were loaded onto an Ni-NTA column. The fractions were run on a 6% polyacrylamide gel and stained with CBB. The eluates of 200 mM imidazole produced major bands at 151 and 135 kDa. Immunoblot patterns of fraction 7 using anti-Myc and anti-HA antibodies are shown on the right. M, molecular weight markers (Bio-Rad); FT, flow-through; Wash, the fractions after washing with 20 mM imidazole. (B) Fraction 7 was fractionated further on a Superose 6 column. The heterodimer Psm1–Psm3 formed a single peak in fractions 22 and 23. CBB bands correspond to the expected molecular weights. (C) AFM micrographs of purified cohesin SMC heterodimer. Bar, 50 nm. (D) Heat-denatured DNA was incubated with either cohesin SMC (Psm1–Psm3), condensin SMC (Cut3-Cut14) or Escherichia coli RecA in buffer A for 0–30 min. After the reaction was terminated by adding SDS, DNAs were run on a 0.7% agarose gel and stained with ethidium bromide (EtBr). The band positions of ssDNA and dsDNA are indicated. (–), ssDNAs without adding proteins. (E) ssDNAs were first incubated for 10 min with or without cohesin SMC (40 or 320 nM). Condensin SMC (40 nM) was then added and incubated further for 0–30 min for reannealing. (–), no addition of cohesin SMC.
The fraction containing Psm1-8Myc and Psm3-3HA-His6 was purified further by gel filtration (Figure 1B). The purity was >95%. As the two proteins co-eluted, they appeared to form an equimolar complex. Gel filtration of the cohesin SMC complex peaked sharply (fractions 22 and 23), in contrast to the broad peak for the condensin SMC heterodimer (data not shown).
AFM of the purified cohesin SMC indicated that the complex had a head and tail appearance (Figure 1C). The tail length of the heterodimer produced two peaks at 25 (left) and 45 nm (middle and right), but the majority had the short (∼25 nm) tail, possibly due to bending (Yoshimura et al., 2002). The coiled-coil tail did not show Y- or V-like structures. Such open structures were observed in the cohesin SMC heterodimer of other species and the bacterial SMC homodimer observed by electron microscopy (Melby et al., 1998; Anderson et al., 2002; Haering et al., 2002), possibly because the resolution of AFM in our hands might be lower than that of electron microscopy. Alternatively, specimens prepared under conditions for AFM may not form an open structure.
Cohesin SMC heterodimer does not promote DNA reannealing
A question addressed was whether the cohesin SMC complex could promote DNA reannealing. Heat-denatured ssDNA (derived from pBluescript, 3 kb) was incubated with or without the cohesin SMC for 0–30 min. The resulting DNA was analysed by agarose gel electrophoresis after the reaction was terminated by the addition of SDS (Figure 1D). The condensin SMC Cut3–Cut14 (Sutani and Yanagida, 1997) and bacterial RecA were employed as control. Unexpectedly, the cohesin SMC did not convert ssDNA into the dsDNA form even after 30 min of incubation (lanes 7–10). For condensin SMC, however, DNA reannealing occurred rapidly and efficiently (Sutani and Yanagida, 1997); within 3 min, the majority of ssDNA was converted into dsDNA, followed by the slow reaction to the high molecular weight form of dsDNA aggregates that remained in the top wells (lanes 11–14). Bacterial RecA also exhibited DNA annealing in the presence of ATP (lanes 15–18), but the efficiency of dsDNA formation was lower than that of the condensin SMC.
The failure of the fission yeast cohesin SMC to induce DNA reannealing was not consistent with previous results for the mammalian recombination complex containing Smc1 and Smc3 that was able to convert ssDNA into dsDNA (Jessberger et al., 1996). We investigated various conditions for reannealing. The concentration of the purified Psm1–Psm3 complex was increased in order to test whether the fission yeast cohesin SMC might contain a weak activity for DNA reannealing. However, reannealing was not detected at all even when the concentration of SMC was raised 3- to 10-fold (data not shown). We then tested several conditions differing in the concentrations of NaCl and other cations, but the cohesin SMC still did not display any reannealing activity.
We then checked whether the cohesin complex could interfere with the reannealing reaction promoted by the condensin complex. For this purpose, reannealing was performed in the presence of the two different kinds of SMC complexes. The cohesin SMC was first added to ssDNA and incubated for 10 min, followed by addition of the condensin SMC. As shown in Figure 1E, the reannealing reaction by the condensin SMC was neither inhibited nor accelerated even in the presence of an eight times higher molar concentration (320 nM) of the cohesin SMC (lanes 9–12) than that (40 nM) of the condensin SMC. We concluded that cohesin SMC neither promoted DNA reannealing nor inhibited condensin SMC during DNA reannealing. This is the first in vitro difference in behaviour between heterodimeric cohesin SMC and condensin SMC.
Cohesin SMC binds slightly but Rad21-bound trimer binds strongly to cruciform DNA
The above results may be explained by the failure of cohesin SMC to interact with ssDNA. We thus examined whether the cohesin SMC heterodimer had the ability to associate with DNA, and the DNA–cellulose binding assay was performed. The unbound (U) and bound (B) proteins were analysed by immunoblotting (Figure 2A). The cohesin SMC showed a moderate binding in the buffer containing 50 mM NaCl. The binding was diminished in the buffer containing 100 mM NaCl, a behaviour distinct from that of condensin SMC heterodimer. Rad21-bound trimer cohesin (see below), however, showed binding in the presence of 100 mM NaCl.
Fig. 2. DNA binding activity of cohesin SMC heterodimer and Rad21-bound heterotrimer. (A) Cellulose coupled with ssDNA (lanes 4, 5, 10 and 11), dsDNA (lanes 6, 7, 12 and 13) or no DNA (lanes 2, 3, 8 and 9) was incubated with cohesin SMC heterodimer (top), condensin SMC heterodimer (middle) or cohesin trimer complex (Psm1–Psm3–Rad21, bottom) in buffer containing 50 mM NaCl (lanes 2–7) or 100 mM NaCl (8–13). Unbound (U) and bound (B) proteins were analysed by immunoblotting using anti-HA (for Psm3 and Cut14) or anti-Psm1 antibodies. (B) AFM micrographs of ssDNA mixed with Psm1–Psm3 complex for 3 min at 30°C (left panel) and ssDNA only (right). Bar, 500 nm. The arrowheads indicate the Psm1–Psm3 particle apparently associated with DNA, while the arrows indicate the Psm1–Psm3 complex free from DNA. (C) Purification of cohesin trimer Psm1, Psm3-3HA and Rad21-8MycHis6. Ni-NTA affinity-purified fractions (Ni) and Superose 6 gel filtration-purified fractions (G.F.) were run on 8% (lanes 1–5) and 6% (lanes 6–8) SDS–polyacrylamide gels and stained with CBB (lane 1–3) or immunoblotted using antibodies against cohesin subunits or the HA or Myc tag added to them (lane 4–8). The void fraction 16 (lanes 2 and 7) and fraction 21 containing the highest concentration of cohesin complex (lanes 3 and 8) are shown. Note that the Rad21 band was hardly observed by CBB. Full-length Rad21 was eluted in fraction 16 (lane 7), whereas the degraded product of Rad21 (indicated by an asterisk) was co-fractionated with Psm1 and Psm3 in fraction 21 (lane 8). (D) Cohesin SMC heterodimer, cohesin trimer complex or condensin SMC heterodimer was mixed with the 32P-labelled short dsDNA (left) or cruciform DNA (right), and subjected to native gel electrophoresis followed by autoradiography. Five femtomoles of labelled DNA were incubated with increasing amounts of protein complex (from left to right for each complex; 0.033, 0.1, 0.3 and 0.9 pmol, respectively). The arrowhead indicates free DNA, while the asterisk shows the top of the gel. Structures of the cruciform and linear dsDNA probes are schematized on the bottom. Linear dsDNA has sequence identical to the north/west arm of the cruciform DNA.
The mixture of ssDNA (linearized and denatured pBluescript) and cohesin SMC was observed by AFM. Reannealed dsDNA was hardly seen (Figure 2B, left panel). A small population of ssDNA seemed to be bound to cohesin SMC (arrowheads). Free SMC particles are indicated by the arrows. A longer incubation time with ssDNA did not increase the frequency of ssDNA bound to cohesin SMC.
Simultaneous overexpression of Rad21, Psm1 and Psm3 allowed us to purify the trimer cohesin (in S.pombe, Psc3 was hardly bound to the complex) by an Ni column followed by gel filtration. Fractions 16 and 21 contained the trimer in abundance (CBB staining, left of Figure 2C) judging by immunoblot patterns (right). The band of Rad21 was diffuse and could hardly be seen by CBB staining. Rad21 was cleaved in fraction 21 during purification. This heterotrimer did not reanneal ssDNA at all like the heterodimeric cohesin SMC (Figure 4A).
Fig. 4. Interaction of condensin with ssDNA and of cohesin with chromatin. (A) The DNA reannealing assay of condensin SMC heterodimer, non-SMC (Cnd) trimer, heteropentameric condensin holo-complex and cohesin trimeric complex in the presence of ATP. See text. (B) AFM images of the mixture of ssDNA and dsDNA (top) and the mixed DNAs with condensin SMC heterodimer (bottom). Bar, 500 nm. (C) Interaction of cohesin SMC heterodimer (Psm1–Psm3) and Rad21-bound heterotrimeric cohesin with reconstituted chromatin was observed by AFM. The filled triangles indicate ‘beads on a string’ of reconstituted chromatin, while the open triangles indicate the aggregated chromatin bound by cohesin trimer. Control circular dsDNA mixed with cohesin trimer is shown on the left. See text for explanation. Bar, 200 nm.
We then examined whether the cohesin heterodimer and hetrotrimer could associate with a short 50 nucleotide piece of dsDNA (Figure 2D, left panel). To this end, a gel retardation assay was performed; 5 fmol end-labelled dsDNA was incubated with four different concentrations (0.033–0.9 pmol) of the complexes in a total of 20 µl of binding buffer. After incubation at room temperature for 1 h, the mixtures were run on native gel electrophoresis (the arrowhead and the asterisk indicate the positions of the unbound DNA and the top of the gel, respectively). Both complexes were scarcely bound to dsDNA, while the control condensin SMC formed the upper bands that did not enter the gel.
If, however, a short piece of cruciform DNA (Bianchi, 1988; Materials and methods) was employed, a significant fraction of the heterotrimer (fractions 21 and 22) could bind and form the smeared upper bands (Figure 2D, right panel). The condensin SMC heterodimer was strongly bound to cruciform DNA. A high affinity for cruciform DNA was known for the C-terminus of SMC (Akhmedov et al., 1998) and the condensin holo-complex (Kimura and Hirano, 1997).
Single SMC, non-SMC heterotrimer and heteropentameric holo-condensin have different binding affinities for cruciform and dsDNA
The above results showed that the ability to promote DNA annealing was specific to the condensin SMC heterodimer at least in fission yeast under the experimental conditions used. To understand how the condensin SMC complex could anneal ssDNAs rapidly, functional dissection of the complex was necessary. It was shown previously that individual condensin SMC subunits failed to promote DNA reannealing (Sutani and Yanagida, 1997). We hence tested whether the individual subunits can bind to DNA, and performed the gel retardation assay using dsDNA and cruciform DNA for the binding substrates (Figure 3A and B).
Fig. 3. DNA binding of condensin single SMC, SMC heterodimer, non-SMC heterotrimer and holo-complex. DNA binding was analysed by electrophoretic mobility shift assay (Figure 2D). (A and B) Binding of single Cut3, Cut14 and the Cut3–Cut14 complex to linear dsDNA or cruciform DNA. (C and D) Binding of Cut3–Cut14 complex, non-SMC trimer and heteropentamer to linear dsDNA or cruciform DNA. DNA used for each reaction was 10 fmol (A and B) or 5 fmol (C and D), and the amounts (molar ratio to DNA) of proteins used are indicated in the figure.
Cut3/Smc4 and Cut14/Smc2 were overexpressed singly in S.pombe, and purified using an Ni column followed by gel filtration. The results of gel retardation somewhat resembled that of the heterodimer, but the degree of binding was considerably different. Cut3 hardly binds to dsDNA, while Cut14 can bind to dsDNA when the protein concentration is high (Figure 3A). The binding of single Cut3 and Cut14 occurs to cruciform DNA (Figure 3B). However, the Cut3–Cut14 heterodimer was even stronger in binding to both dsDNA and cruciform DNA. The ability of SMC to bind DNA was thus not sufficient to promote DNA reannealing.
Another question was whether non-SMC trimer (Sutani et al., 1999) was able to interact with DNA. The trimer has a globular shape and interacts with the head domains of SMC heterodimer to form the holo-complex (Anderson et al., 2002; Yoshimura et al., 2002). For gel retardation assay, the cruciform and dsDNA were employed. As shown in Figure 3C and D, the non-SMC trimer did not seem to form any stable complex with DNAs. The non-SMC trimer itself thus had very weak, if any, DNA-binding activity, as is the case for the frog non-SMC trimer (Kimura and Hirano, 2000).
The gel shift assay was then applied to the holo-condensin complex, and the results are shown in Figure 3C and D. The procedures used to prepare the whole condensin were described previously (Yoshimura et al., 2002). Interaction with dsDNA was weak, and the faint upper band was observed when the maximal protein concentration was employed (indicated by the arrow). Cruciform DNA had stronger affinity for holo-condensin, but the binding efficiency was weaker in comparison with that for SMC heterodimer. In these experiments, the presence or absence of ATP had little effect on gel retardation for both complexes (data not shown). These results are somewhat different from the frog system (Kimura and Hirano, 2000). The frog 13S condensin had a higher affinity for DNA than heterodimer SMC. This may be due to the different cell cycle stages from which the condensin specimens were derived (frog from M phase, and fission yeast largely from G2 phase).
The heteropentameric condensin weakly promotes but heterotrimeric cohesin does not promote DNA annealing
We wanted to know whether the condensin holo-complex was capable of promoting DNA annealing. Using the condensin holo-complex purified by affinity chromatography, DNA reannealing was examined in the presence or absence of 1 mM ATP (Figure 4A; data not shown). The reannealing activity of the condensin holo-complex was weak, regardless of the presence or absence of ATP. The rate of conversion from ssDNA to dsDNA was faster than that of DNA self-annealing but much slower than that with heterodimeric SMC. The high molecular weight DNAs produced in the upper wells by condensin SMC were not produced at all. The non-SMC trimer might inhibit the reannealing activity of the condensin SMC heterodimeric complex. Indeed, the non-SMC trimer itself showed no reannealing activity at all. We then employed the cohesin trimer containing Rad21 and found that it did not promote DNA reannealing. The ability to promote DNA reannealing thus specifically resided in condensin.
As the substrate of the DNA reannealing reaction is ssDNA, we assessed whether the condensin SMC complex had stronger affinity for ssDNA than dsDNA by AFM observation of the mixtures of ssDNA, dsDNA and condensin SMC heterodimer (Figure 4B). The heterodimeric condensin was bound selectively to ssDNA (bottom panel), indicating that the condensin heterodimer has stronger affinity for ssDNA than dsDNA.
Cohesin complex binds to chromatin
We next examined whether cohesin SMC heterodimer or trimer can bind to chromatin reconstituted in vitro using histone octamer prepared from HeLa cells and pBluescript supercoiled circular DNA. AFM images of the mixtures were taken (Figure 4C). The images of chromatin itself revealed a feature of ‘beads on a string’ (filled arrowheads). The SMC heterodimer (Psm1–Psm3) did not seem to interact with the chromatin, producing the same structural feature as chromatin alone. The addition of cohesin trimer, however, caused a dramatic change in chromatin, which became more compact and dense (open arrowheads), suggesting that cohesin trimer containing Rad21 might bind to chromatin. Binding of cohesin trimer to naked DNA was rare (left panel).
Condensin heterodimer containing one terminally deleted SMC can promote DNA reannealing
To understand the minimal structure required for the reannealing activity, a series of truncated Cut3 and Cut14 forms were constructed (Figure 5A) and co-overproduced in order to form complexes containing truncated polypeptides. For instance, the complex consisting of full-length Cut14 (tagged with 2HA-His6) and Cut3ΔNC (tagged with 8Myc) lacking both N- and C-terminal globular domains is shown in Figure 5B (lane 2). Cut3ΔNC–Cut14 and reciprocally truncated Cut3–Cut14ΔNC, respectively, were mixed with ssDNA. Both were able to promote DNA annealing rapidly (Figure 5C, Full-ΔNC and ΔNC-Full). The somewhat slow reannealing rate of ΔNC-Full may not be significant, as in other experiments the ΔNC-Full promoted annealing as efficiently as full-length Cut3–Cut14 (data not shown). The terminal globular domains in one SMC are thus dispensable for DNA reannealing.
Fig. 5. Truncated SMC heterodimers are still able to anneal ssDNA. (A) Various terminally truncated Cut3 and Cut14 forms were made. The number indicates the amino acid residue where truncation took place. Truncated forms of Cut3 (or Cut14) were expressed in combination with co-overexpression of the wild-type or truncated forms of Cut14 (or Cut3). (B) One example of the purified truncated complex is shown. Cut14 is bound to the terminally truncated Cut3ΔNC-8Myc lacking both the N- and C-termini. Lanes 1 and 2 in the SDS–polyacrylamide gel stained by CBB represent the wild-type Cut3–Cut14 complex and the truncated complex, respectively. The wild-type Cut3 is highly sensitive to protease during preparation, yielding two bands (Sutani and Yanagida, 1997). (C) DNA reannealing activity remains as strong in Cut3–Cut14ΔNC and Cut3ΔNC–Cut14 as the wild-type combination. In other combinations, the reannealing activity was either null or very weak. (D) Gel shift experiment indicating that Cut3ΔNC–Cut14ΔN that did not promote DNA reannealing was still able to associate with cruciform DNA. The full-length Cut3–Cut14 complex was used as control.
One set of globular domains has to be derived from the same SMC
The truncated heterodimer Cut3ΔNC–Cut14ΔN that consists of Cut3 lacking both the N- and C-termini, and Cut14 lacking the N-terminus failed to promote DNA reannealing (Figure 5C, ΔNC-ΔN). One set of globular domains must exist in order to promote reannealing. This is consistent with other findings that two different constructs Cut3ΔN–Cut14ΔN (ΔN-ΔN) and Cut3ΔC–Cut14ΔN (ΔC-ΔN) produced little DNA reannealing (Figure 5C, two rightmost panels). Cut3ΔN–Cut14ΔC (ΔN-ΔC) and Cut3ΔC–Cut14ΔC (ΔC-ΔC) showed weak activity for DNA reannealing (data not shown). Thus, one set of the globular domains has to be derived from the same SMC, regardless of whether it is Cut3/Smc4 or Cut14/Smc2. This conclusion is important as the head domains of Cut3 and Cut14 carry a similar function to promote reannealing.
Gel retardation assay showed that Cut3ΔNC–Cut14ΔN was still bound to the cruciform DNA (Figure 5D), indicating that the ability to bind to DNA that remained in the complex was not sufficient for reannealing. The ability of Cut3ΔNC–Cut14ΔN to bind to ssDNA and dsDNA was also confirmed by DNA–cellulose binding assay (data not shown). As efficient binding to DNA occurred for Cut3ΔNC–Cut14ΔN, the two abilities of condensin heterodimer SMC, DNA binding and reannealing, can be separated. The hinge domain seemed to play a role in dimerization as reported for cohesin heterodimer (Haering et al., 2002), as SMC not containing the hinge region did not form the complex (data not shown). The hinge region may have specificity for one SMC subunit to interact with the other.
Intermediary protein–DNA complexes formed before the completion of re-annealing
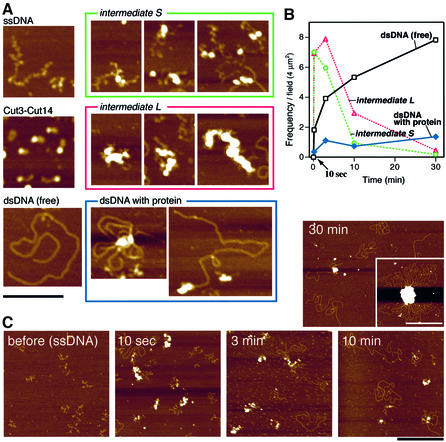
To gain information on the mechanistic aspect of DNA reannealing by condensin SMC, the time course analysis was performed by AFM. Micrographs were taken for the mixture containing ssDNA (5 nM) and condensin SMC heterodimer (45 nM) after 10 s, 3, 10 and 30 min at 25°C. Figure 6A (left panels) shows denatured linear ssDNA (pBluescript, 3 kb), purified condensin SMC heterodimer and renatured dsDNA. The random coil-like feature of ssDNA is easily distinguished from that of dsDNA, which appears as continuous filaments. At 10 s and 3 min after mixing, two types of intermediary structures S and L were seen in abundance (right panels). Quantitative data are shown in Figure 6B. Intermediate S seems to consist of ssDNA bound to a small number of SMCs, while intermediate L is heavily associated with the protein complexes. Since DNA reannealing was already rather advanced within 3 min (see Figure 1D) even at 25°C, a number of dsDNA molecules were observed together with the intermediary structures after 3 min. Images of dsDNAs become abundant 3 and 10 min after mixing. A subfraction of dsDNA was associated with the protein complex at the ends of dsDNA (Figure 6A, right bottom panel). Low magnification images at each time point are shown in Figure 6C.
Fig. 6. Time course analysis of DNA reannealing by condensin SMC heterodimer. (A) Left panel: AFM images of ssDNA (denatured ssDNA of linearized 3 kb pBluescript), Cut3–Cut14 (the purified condensin SMC heterodimer) and linear dsDNA. Right panel: (top) intermediate S (reaction intermediate containing protein particles sparsely bound to ssDNA); (middle) intermediate L (reaction intermediates showing large protein aggregates associated with DNA; and (bottom) reannealed dsDNA bound by protein. Left: Spool-like dsDNA with protein in the centre. Right: dsDNA with protein at one end. Bar, 200 nm. (B) Time course analysis of the appearance of four distinct structures; intermediate S and L, and dsDNA with and without bound protein. The number of each type of particles observed in one individual scan area (2 µm × 2 µm) was counted (total of 11–19 scans areas examined for each time point). (C) Low magnification images of ssDNA before the reaction (left), and 10 s (centre left), 3 min (centre right), 10 min (right) and 30 min (right top) after mixing with condensin SMC heterodimer and incubation at 25°C. The inset in the micrograph of 30 min represents a large protein–DNA aggregate (see text). Bars, 500 nm.
As the features of ssDNA remained, intermediate S should represent an earlier stage of reannealing than intermediate L. This was consistent with the fact that the peak for intermediate S was at 10 s while that for L was at 3 min (Figure 6B). In intermediate L, the DNAs protruding looked as if they were still ssDNA, suggesting that intermediate L was at a stage before completion of DNA annealing. The dsDNA images were only seen infrequently with the protein assembly. We hypothesized that intermediate L represented partially annealed DNAs heavily associated with SMC heterodimers.
The dsDNA structures associated with protein complexes sometimes revealed peculiar concentric spool-like structure. Proteins may be associated with the ends of filaments. Consistently, mature dsDNA filaments are in some cases associated with the protein complex at their end. Most renatured DNAs were largely devoid of proteins. After further incubation for 30 min, very large complexes were observed occasionally with protein aggregates (Figure 6C, right top inset). These large structures were made slowly after the fast reannealing process as they were hardly seen in the 10 s and 3 min preparations. The high molecular weight forms of DNAs seen in agarose gel electrophoresis might be derived from these large structures.
Discussion
In this study, various complexes of condensin and cohesin were purified from fission yeast cells and found to behave very differently in their interaction with DNA. The ability to reanneal ssDNA only applies to condensin. Even for highly concentrated cohesin SMC heterodimer, no trace of reannealing-enhancing activity was detected, in sharp contrast to the extremely fast reannealing activity possessed by condensin SMC heterodimer. The failure of cohesin SMC to promote DNA reannealing was unexpected because mammalian RC-1 containing the SMC1–SMC2 complex (now assigned as SMC1–SMC3) was shown to promote DNA reannealing (Jessberger et al., 1996). The reason for this discrepancy was unclear, and was possibly due to species differences. Note that the data for DNA reannealing by RC-1 are relatively rather weak in comparison with those of condensin SMC heterodimer. To our knowledge, this is the first evidence that the SMC heterodimers of condensin and cohesin exhibit different molecular activities, at least in one species. Non-SMC subunits have been considered to confer specificity for the in vivo roles of cohesin and condensin, but the SMC heterodimers themselves may also carry specific functions.
A particular property of SMC may be involved in this unexpected specificity. The helix-breaking residues are more plentiful in the coiled-coil regions (13 proline residues) of cohesin SMC than those (six prolines) of condensin SMC. The coiled-coil region of cohesin SMC might be more flexible than that of condensin. Coiled-coil regions in cohesin and bacterial SMC homodimer appear to be open as V- or Y-shaped structures in electron microscopy (Melby et al., 1998; Anderson et al., 2002; Haering et al., 2002), or frequently bent in AFM (Figure 1C). The amino acid sequences of the hinge regions of SMC are distinct between cohesin and condensin, and also unique for each SMC. These sequences, which are evolutionarily conserved, may be related to different abilities for DNA reannealing.
Cohesin SMC heterodimer interacts weakly with linear dsDNA and cruciform DNA. The heterotrimeric Rad21-bound SMC complex also showed weak interaction with dsDNA but interacted relatively strongly with cruciform DNA. Cohesin may not have to interact strongly with dsDNA to make the link between the two dsDNAs, as it may have a ring-like structure to bring two duplex DNAs together (Haering et al., 2002). HP-1, like Swi6, was found to be required for recruiting cohesin to heterochromatic regions (Bernard et al., 2001; Nonaka et al., 2002). Swi6 may recruit cohesin to the site where sister chromatid cohesion should be established. Alternatively, the cohesin trimer has affinity for the higher order DNA structure, which might exist in chromatin DNA but not in regular dsDNA. Consistently, the trimer cohesin was bound to reconstituted chromatin, which contained constrained DNA. Another possibility is that cohesin trimer has affinity for histones. Frog cohesin was shown to have strong affinity for supercoiled DNA (Losada and Hirano, 2001).
The fact that the strong activity in DNA reannealing is particular to condensin SMC heterodimer needs to be interpreted in physiological terms. The fact that an SMC mutant protein failed to promote DNA reannealing (Sutani and Yanagida, 1997) was evidence that the activity might be implicated in condensin function in vivo. The mutation site was near the hinge region essential for condensation. Truncation experiments and the findings that single SMC was not enough for the activity strongly suggested that the heterodimeric structure of the hinge and/or the coiled-coil regions was necessary for DNA reannealing, as schematized in Figure 7A. Additionally, one set of the globular domains from the same SMC was necessary for rapidly reannealing DNA. In the heteropentameric condensin that had a rather weak reannealing activity (Figure 4A), the non-SMC trimeric complex may mask the ability of heterodimer SMC by associating with the globular domains (Anderson et al., 2002; Yoshimura et al., 2002). In contrast to cohesin complexes which have no reannealing activity at all, heteropentameric holo-condensin does show a weak activity without forming the large DNA aggregates, strongly suggesting that the reannealing activity is specific to condensin. The reannealing activity is possibly partial, transient or cryptic for the in vivo holo-condensin function. Alternatively, the activity could be revealed strongly in M phase or whenever it became necessary.
Fig. 7. Model for DNA reannealing. (A) Summary of the truncated condensin SMC heterodimer that was capable of reannealing. +, reannealing positive; –, reannealing negative or weak. (B) A model diagram for the steps of ssDNA reannealing by condensin SMC heterodimer. AFM micrographs of corresponding stages are shown. Protein assembly on ssDNA is a principal feature of intermediate structures. See text for explanation. Complementary ssDNAs are indicated in different colours.
The non-SMC heterotrimer Cnd1–Cnd2–Cnd3 hardly binds to dsDNA and cruciform DNA, whereas the holo-condensin complex showed substantial DNA binding that was still weaker than that of condensin SMC heterodimer (Figure 3). This fact may be important for considering how condensin interacts with chromosome structure, as little is known about how non-SMC subunits are mechanistically implicated in in vivo condensin function. Non-SMC trimer may bind to the globular domains of SMC and inhibit strong interaction with DNA (Yoshimura et al., 2002). Frog non-SMC prepared from M phase binds weakly to DNA, and DNA binding of SMC heterodimer is enhanced by non-SMC trimer (Kimura and Hirano, 2000). This is different from the case of fission yeast. The reason for this difference remains to be understood.
The time course analysis of the annealing reaction seen by AFM revealed the steps of intermediary SMC protein–ssDNA assembly. The intermediary complexes seemed to consist of assembled SMC protein and ssDNA. This large protein aggregate bound to DNA is an unexpected feature as an intermediary protein–DNA complex for reannealing. We propose a hypothesis that protein–protein interaction among SMC heterodimers induces DNA reannealing (Figure 7B). This model is consistent with the fact that the high concentration of condensin SMC (∼10-fold) relative to ssDNA is needed for annealing: no reannealing occurs at all if the concentration of SMC is low (Sutani and Yanagida, 1997). The stage should be intermediate as the completed dsDNAs were mostly devoid of protein, except for occasional association of protein at the end of dsDNA. As heterodimer SMC had selective affinity for ssDNA rather than dsDNA (Figure 4B), it would easily fall off from reannealed dsDNA. If the incubation time is prolonged, however, large DNA aggregates bound to protein slowly form (Figure 6A, 30 min inset). These DNA aggregates that do not enter the gel are probably the product of a reaction other than reannealing. It was hardly found after 3 min when virtually the maximal reannealed dsDNA was made. As DNA aggregates disappear after S1 nuclease digestion (Sutani and Yanagida, 1997), they might possibly be formed by multiple strand joining of dsDNA, which should be susceptible to S1 nuclease.
The coiled-coil and hinge regions are required for initial binding to DNA, as the truncated form lacking three of the four globular domains can still bind to DNA. This is consistent with the findings that coiled-coil regions of SMC from other organisms can bind to DNA (Akhmedov et al., 1999; Hirano and Hirano, 2002). The truncated form lacking all of the four globular domains of two SMC subunits was highly susceptible to proteolysis and not able to be tested. How are two complementary strands recognized and bound? There may be two possibilities. Recognition of the two complementary strands does not require SMC protein, but the collision rate between the two ssDNA strands could be increased through compacting ssDNA by the bound SMC. Alternatively, the SMC heterodimer efficiently guides two complementary strands through its long coiled-coil region and/or the large protein–protein assembly formed on DNA. In either case, the SMC heterodimers may have to be assembled on DNA in order to initiate the fast reannealing through either compaction of ssDNA or association of plus and minus strands (Figure 7B).
The bacterial SMC homodimer which has DNA reannealing activity also exhibits DNA-dependent protein–protein interaction (Hirano and Hirano, 1998; Hirano et al., 2001). DNA reannealing by prokaryotic and eukaryotic SMC thus seemed to require protein–protein interaction. Since cohesin SMC does not have the ability to reanneal DNA, bacterial SMC resembles condensin SMC rather than cohesin SMC. Bacterial chromosome DNA must be compacted to exist in nucleoids, but may not have to hold together duplicated sister chromosome DNAs as there is no obvious G2 phase and the mitotic step for anaphase chromosome segregation.
What would be the actual functional implication of DNA reannealing by condensin SMC heterodimer? There is no known step in condensation that apparently requires the association of complementary DNA strands. The fact that nuclear chromatin obtained from cut14 mutant cells was highly sensitive to S1 nuclease (Sutani and Yanagida, 1997), however, suggests the possible role of the condensin complex in processing ssDNA in vivo during mitotic chromosome condensation. Furthermore, a recent study by Aono et al. (2002) showing that condensin has an interphase function related to recovery from the S phase interference or activation of the replication checkpoint kinase Cds1/Chk2 sheds light on the possible in vivo role of DNA reannealing by condensin SMC. For condensin, the DNA annealing activity might be required for the recovery from unwound duplex DNAs. Complementary DNA strands in chromosome are thought to be always spontaneously associated but, in certain situations, the DNA reannealing reaction might have to be positively supported. Even during mitotic chromosome condensation, DNA reannealing might be needed.
Materials and methods
Plasmids
The cohesin SMC genes psm1+ and psm3+ (Tomonaga et al., 2000) were subcloned into pREP1 (LEU2 marker) and pREP2 (ura4+ marker), respectively, and expressed under the nmt1 promoter (Maundrell, 1993). The C-termini of psm1+ and psm3+ were tagged with 8Myc and 3HA-His6, respectively. To express Cut3ΔNC (amino acids 279–1206), the SmaI–BamHI fragment of cut3+ was subcloned into pREP1 using NdeI and NotI linkers. The fragment encoding Cut14ΔNC (amino acids 170–1043) was amplified by PCR and subcloned into pREP2. All of the plasmid constructs were verified by sequencing. Various Cut3 constructs are tagged with 8Myc at the C-termini, whereas Cut14 constructs are tagged with 3HA-His6 at the C-termini.
Overproduction and protein purification
Simultaneous overproduction and purification of single SMC (Cut3 or Cut14), sub- and holo-complexes of condensin and cohesin was carried out as described (Sutani and Yanagida, 1997; Yoshimura et al., 2002) with some modifications. Cells carrying plasmids for overproduction were cultured in EMM2 medium in the absence of thiamine for 14–16 h at 33°C or for 24–30 h at 26°C. The buffers used for extraction were Ext (40 mM Tris–HCl pH 7.5, 200 mM NaCl and 10% glycerol) for Psm1–Psm3, and HB (25 mM Tris–HCl pH 7.5, 50 mM NaCl, 15 mM MgCl2, 60 mM β-glycerophosphate and 0.1% NP-40 plus protease inhibitors) for truncated Cut3–Cut14. Purified preparations were concentrated with Microcon 50 (Amicon).
DNA reannealing assay
The DNA reannealing assay was carried out described (Sutani and Yanagida, 1997). For the reaction containing ATP, buffer A [20 mM Tris–HCl pH 7.5, 50 mM NaCl, 10 mM MgCl2, 1 mM ATP, 1 mM dithiothreitol (DTT), 10% glycerol] was used. ATP-free reactions were performed in buffer B (20 mM Tris–HCl pH 7.5, 100 mM NaCl, 1 mM DTT, 10% glycerol). RecA protein was a gift from Dr T.Horii.
DNA cellulose binding assay
Protein fractions (80 µM) were incubated at room temperature for 15 min with control cellulose (Sigma; 10 µl bed), coupled ssDNA or dsDNA in 40 µl of buffer D [20 mM Tris–HCl pH 8.0, 1 mM EDTA, 10% glycerol, 0.02% NP-40, 1 mM DTT, 0.1 mM phenylmethylsulfonyl fluoride (PMSF)] containing either 50 or 100 mM NaCl. After washing the cellulose with the same buffer, unbound and bound proteins were analysed by immunoblot.
Gel retardation assay
32P-Labelled DNA was incubated with the indicated amounts of protein complex (gel filtration-purified fractions) in a total of 20 µl in binding buffer [20 mM Tris–HCl pH 7.5, 50 mM NaCl, 2 mM MgCl2, 10% glycerol, 1 mM DTT, 100 µg/ml bovine serum albumin (BSA)] for 1 h at room temperature. The samples were run at 4°C on either 5% (for cruciform DNA) or 8% (for dsDNA) non-denaturing polyacrylamide gels made in TBE, followed by autoradiography. The cruciform DNA and control linear duplex DNA used in this assay were made and purified as described (Bianchi, 1988). In brief, four oligonucleotides of 46–59 nucleotides in length constructed to produce cruciforms were annealed in a buffer (10 mM Tris–HCl pH 7.5, 50 mM NaCl, 10 mM MgCl2, 1 mM DTT), followed by polyacrylamide gel purification. The control linear duplex DNA had one of the four oligonucleotides used to make the cruciform DNA. The oligonucleotide was 32P end-labelled prior to annealing.
AFM imaging
AFM imaging was performed as described (Yoshimura et al., 2002). For the reconstitution of chromatin, 15 ng of core histone and 15 ng of pBluescript were mixed in buffer C (10 mM Tris–HCl pH 7.5, 5 mM 2-mercaptoethanol, 0.05% NP-40, 0.1 mM PMSF) containing 2 M NaCl and dialysed against buffer C to obtain the final concentration of 50 mM NaCl. The reconstituted chromatin was mixed with Psm1–Psm3 or cohesin trimeric complex (10 ng per subunit) and samples were fixed with 0.3% glutaraldehyde (GA) after incubation on ice for 30 min. To observe the time course of DNA reannealing, 30 ng of heat-denatured linear DNA (pBluescript) was incubated with 80 ng of Cut3–Cut14 heterodimer in 5 mM HEPES at pH 7.4 containing 100 mM NaCl in a total of 6 µl at 25°C. After 10 s, 3, 10 or 30 min, the reaction was terminated by adding the same volume of ice-cold 0.2% GA. Samples were fixed for 1.5 h on ice and diluted with 5 mM HEPES pH 7.4 before depositing on mica. For AFM imaging, 1/10 volume of the reaction mixture was applied on the freshly cleaved mica surface pre-treated with 10 mM spermidine. After 10 min, the mica was washed with water and dried with nitrogen gas. Samples were observed in air at room temperature using Nanoscope IIIa (Digital Instruments) with a type E scanner under the Tapping Mode.
Acknowledgments
Acknowledgements
We thank S.H.Yoshimura for providing an AFM micrograph of cohesin SMC heterodimer. This work was supported by a CREST Research Grant of the Japan Science and Technology Corporation (JST) and a COE Grant of the Ministry of Education, Culture, Sports, Science and Technology of Japan. T.S. was a recipient of the fellowship of the Japan Science Promotion Society (JSPS).
References
- Akhmedov A.T., Frei,C., Tsai-Pflugfelder,M., Kemper,B., Gasser,S.M. and Jessberger,R. (1998) Structural maintenance of chromosomes protein C-terminal domains bind preferentially to DNA with secondary structure. J. Biol. Chem., 273, 24088–24094. [DOI] [PubMed] [Google Scholar]
- Akhmedov A.T., Gross,B. and Jessberger,R. (1999) Mammalian SMC3 C-terminal and coiled-coil protein domains specifically bind palindromic DNA, do not block DNA ends and prevent DNA bending. J. Biol. Chem., 274, 38216–38224. [DOI] [PubMed] [Google Scholar]
- Anderson D.E., Losada,A., Erickson,H.P. and Hirano,T. (2002) Condensin and cohesin display different arm conformations with characteristic hinge angles. J. Cell Biol., 156, 419–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aono N., Sutani,T., Tomonaga,T., Mochida,S. and Yanagida,M. (2002) Cnd2 has dual roles in mitotic condensation and interphase. Nature, 417, 197–202. [DOI] [PubMed] [Google Scholar]
- Bernard P., Maure,J.F., Partridge,J.F., Genier,S., Javerzat,J.P. and Allshire,R.C. (2001) Requirement of heterochromatin for cohesion at centromeres. Science, 294, 2539–2542. [DOI] [PubMed] [Google Scholar]
- Bianchi M.E. (1988) Interaction of a protein from rat liver nuclei with cruciform DNA. EMBO J., 7, 843–849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cobbe N. and Heck,M.M. (2000) Review: SMCs in the world of chromosome biology—from prokaryotes to higher eukaryotes. J. Struct. Biol., 129, 123–143. [DOI] [PubMed] [Google Scholar]
- Freeman L., Aragon-Alcaide,L. and Strunnikov,A. (2000) The condensin complex governs chromosome condensation and mitotic transmission of rDNA. J. Cell Biol., 149, 811–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haering C.H., Lowe,J., Hochwagen,A. and Nasmyth,K. (2002) Molecular architecture of SMC proteins and the yeast cohesin complex. Mol. Cell, 9, 773–788. [DOI] [PubMed] [Google Scholar]
- Hagstrom K.A., Holmes,V.F., Cozzarelli,N.R. and Meyer,B.J. (2002) C.elegans condensin promotes mitotic chromosome architecture, centromere organization and sister chromatid segregation during mitosis and meiosis. Genes Dev., 16, 729–742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirano M. and Hirano,T. (1998) ATP-dependent aggregation of single-stranded DNA by a bacterial SMC homodimer. EMBO J., 17, 7139–7148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirano M. and Hirano,T. (2002) Hinge-mediated dimerization of SMC protein is essential for its dynamic interaction with DNA. EMBO J., 21, 5733–5744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirano M., Anderson,D.E. and Hirano,T. (2001) Bimodal activation of SMC ATPase by intra- and inter-molecular interactions. EMBO J., 20, 3238–3250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirano T. (2000) Chromosome cohesion, condensation and separation. Annu. Rev. Biochem., 69, 115–144. [DOI] [PubMed] [Google Scholar]
- Hirano T. (2002) The ABCs of SMC proteins: two-armed ATPases for chromosome condensation, cohesion and repair. Genes Dev., 16, 399–414. [DOI] [PubMed] [Google Scholar]
- Hirano T., Kobayashi,R. and Hirano,M. (1997) Condensins, chromosome condensation protein complexes containing XCAP-C, XCAP-E and a Xenopus homolog of the Drosophila Barren protein. Cell, 89, 511–521. [DOI] [PubMed] [Google Scholar]
- Jessberger R., Riwar,B., Baechtold,H. and Akhmedov,A.T. (1996) SMC proteins constitute two subunits of the mammalian recombination complex RC-1. EMBO J., 15, 4061–4068. [PMC free article] [PubMed] [Google Scholar]
- Kimura K. and Hirano,T. (1997) ATP-dependent positive supercoiling of DNA by 13S condensin: a biochemical implication for chromosome condensation. Cell, 90, 625–634. [DOI] [PubMed] [Google Scholar]
- Kimura K. and Hirano,T. (2000) Dual roles of the 11S regulatory subcomplex in condensin functions. Proc. Natl Acad. Sci. USA, 97, 11972–11977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura K., Hirano,M., Kobayashi,R. and Hirano,T. (1998) Phosphorylation and activation of 13S condensin by Cdc2 in vitro. Science, 282, 487–490. [DOI] [PubMed] [Google Scholar]
- Kimura K., Rybenkov,V.V., Crisona,N.J., Hirano,T. and Cozzarelli,N.R. (1999) 13S condensin actively reconfigures DNA by introducing global positive writhe: implications for chromosome condensation. Cell, 98, 239–248. [DOI] [PubMed] [Google Scholar]
- Losada A. and Hirano,T. (2001) Intermolecular DNA interactions stimulated by the cohesin complex in vitro: implications for sister chromatid cohesion. Curr. Biol., 11, 268–272. [DOI] [PubMed] [Google Scholar]
- Losada A., Hirano,M. and Hirano,T. (1998) Identification of Xenopus SMC protein complexes required for sister chromatid cohesion. Genes Dev., 12, 1986–1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maundrell K. (1990) nmt1 of fission yeast. A highly transcribed gene completely repressed by thiamine. J. Biol. Chem., 265, 10857–10864. [PubMed] [Google Scholar]
- Maundrell K. (1993) Thiamine-repressible expression vectors pREP and pRIP for fission yeast. Gene, 123, 127–130. [DOI] [PubMed] [Google Scholar]
- Melby T.E., Ciampaglio,C.N., Briscoe,G. and Erickson,H.P. (1998) The symmetrical structure of structural maintenance of chromosomes (SMC) and MukB proteins: long, antiparallel coiled coils, folded at a flexible hinge. J. Cell Biol., 142, 1595–1604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasmyth K. (2001) Disseminating the genome: joining, resolving and separating sister chromatids during mitosis and meiosis. Annu. Rev. Genet., 35, 673–745. [DOI] [PubMed] [Google Scholar]
- Nonaka N., Kitajima,T., Yokobayashi,S., Xiao,G., Yamamoto,M., Grewal,S.I. and Watanabe,Y. (2002) Recruitment of cohesin to heterochromatic regions by Swi6/HP1 in fission yeast. Nat. Cell Biol., 4, 89–93. [DOI] [PubMed] [Google Scholar]
- Saka Y., Sutani,T., Yamashita,Y., Saitoh,S., Takeuchi,M., Nakaseko,Y. and Yanagida,M. (1994) Fission yeast cut3 and cut14, members of a ubiquitous protein family, are required for chromosome condensation and segregation in mitosis. EMBO J., 13, 4938–4952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steffensen S. et al. (2001) A role for Drosophila SMC4 in the resolution of sister chromatids in mitosis. Curr. Biol., 11, 295–307. [DOI] [PubMed] [Google Scholar]
- Strunnikov A.V. and Jessberger,R. (1999) Structural maintenance of chromosomes (SMC) proteins: conserved molecular properties for multiple biological functions. Eur. J. Biochem., 263, 6–13. [DOI] [PubMed] [Google Scholar]
- Strunnikov A.V., Hogan,E. and Koshland,D. (1995) SMC2, a Saccharomyces cerevisiae gene essential for chromosome segregation and condensation, defines a subgroup within the SMC family. Genes Dev., 9, 587–599. [DOI] [PubMed] [Google Scholar]
- Sutani T. and Yanagida,M. (1997) DNA renaturation activity of the SMC complex implicated in chromosome condensation. Nature, 388, 798–801. [DOI] [PubMed] [Google Scholar]
- Sutani T., Yuasa,T., Tomonaga,T., Dohmae,N., Takio,K. and Yanagida,M. (1999) Fission yeast condensin complex: essential roles of non-SMC subunits for condensation and Cdc2 phosphorylation of Cut3/SMC4. Genes Dev., 13, 2271–2283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomonaga T. et al. (2000) Characterization of fission yeast cohesin: essential anaphase proteolysis of Rad21 phosphorylated in the S phase. Genes Dev., 14, 2757–2770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toth A., Ciosk,R., Uhlmann,F., Galova,M., Schleiffer,A. and Nasmyth,K. (1999) Yeast cohesin complex requires a conserved protein, Eco1p(Ctf7), to establish cohesion between sister chromatids during DNA replication. Genes Dev., 13, 320–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhlmann F., Lottspeich,F. and Nasmyth,K. (1999) Sister-chromatid separation at anaphase onset is promoted by cleavage of the cohesin subunit Scc1. Nature, 400, 37–42. [DOI] [PubMed] [Google Scholar]
- Uhlmann F., Wernic,D., Poupart,M.A., Koonin,E.V. and Nasmyth,K. (2000) Cleavage of cohesin by the CD clan protease separin triggers anaphase in yeast. Cell, 103, 375–386. [DOI] [PubMed] [Google Scholar]
- Yoshimura S.H., Hizume,K., Murakami,A., Sutani,T., Takeyasu,K. and Yanagida,M. (2002) Condensin architecture and interaction with DNA: regulatory non-SMC subunits bind to the head of SMC heterodimer. Curr. Biol., 12, 508–513. [DOI] [PubMed] [Google Scholar]