Abstract
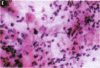
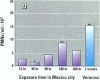
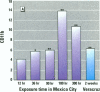
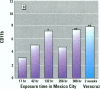
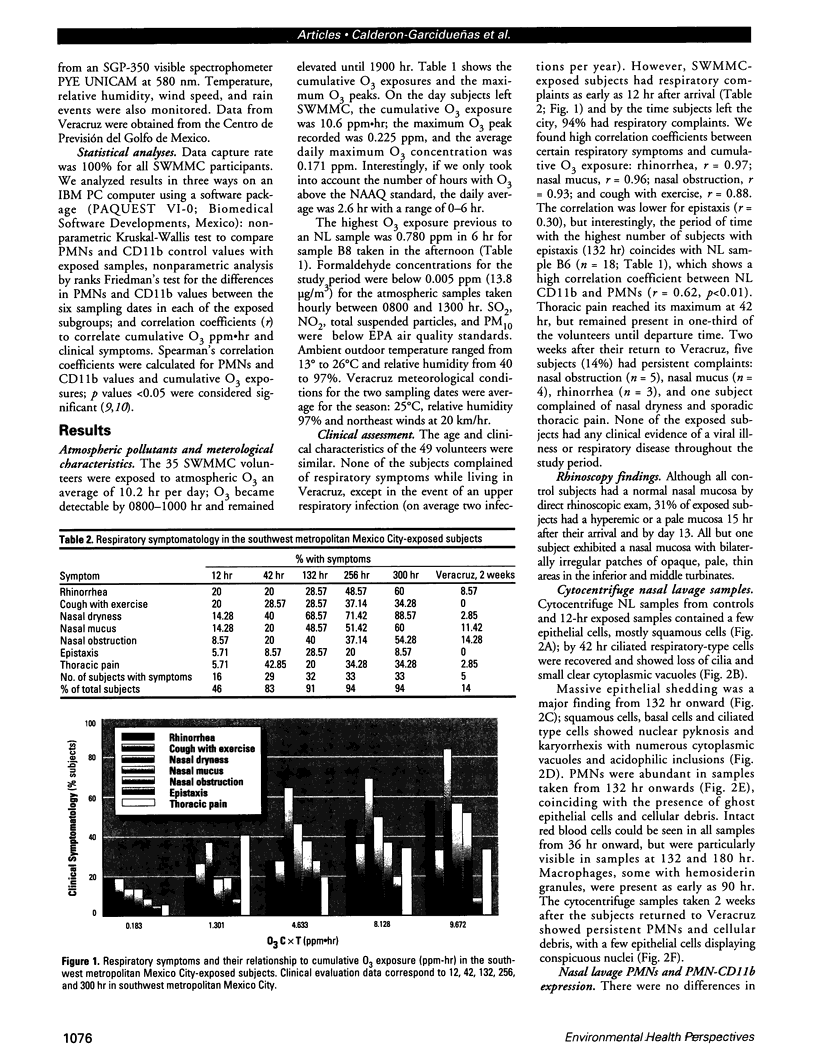
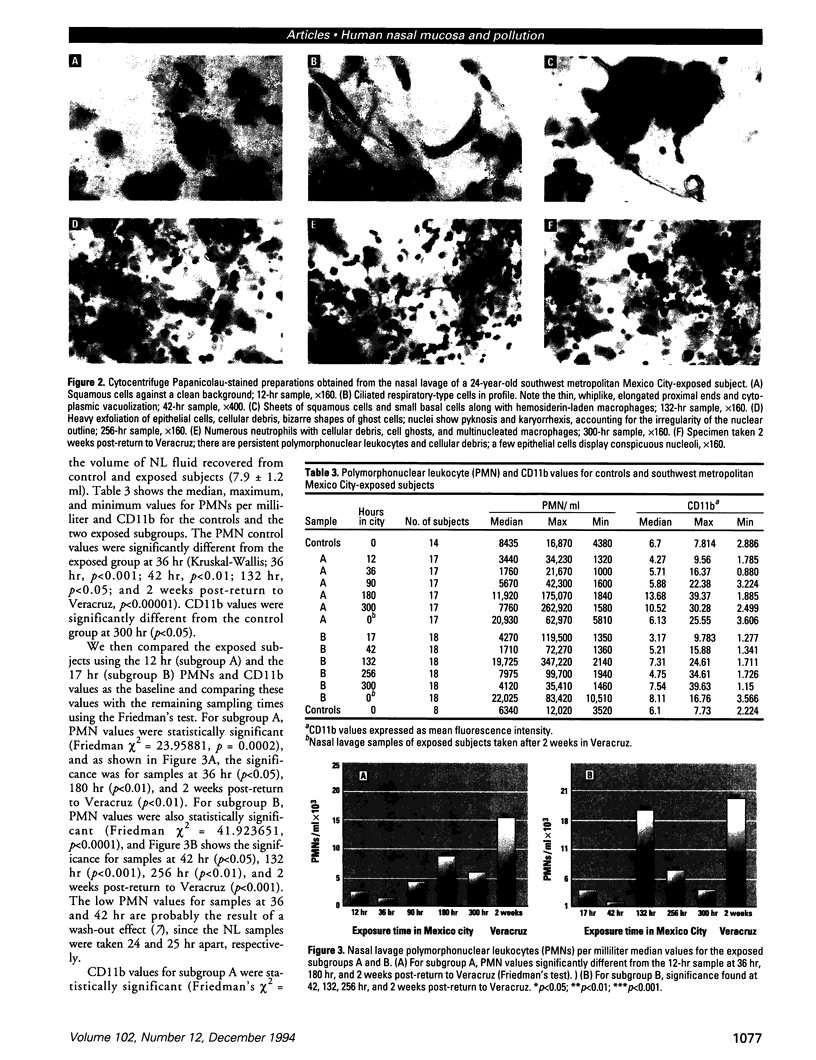
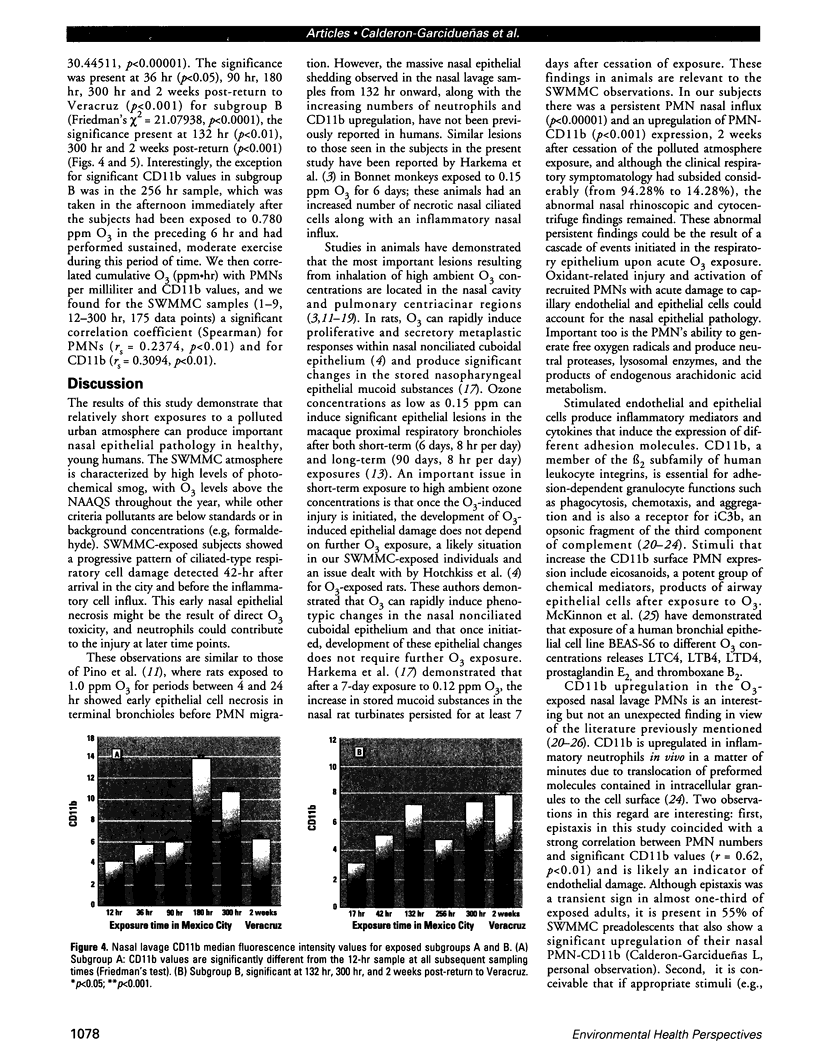
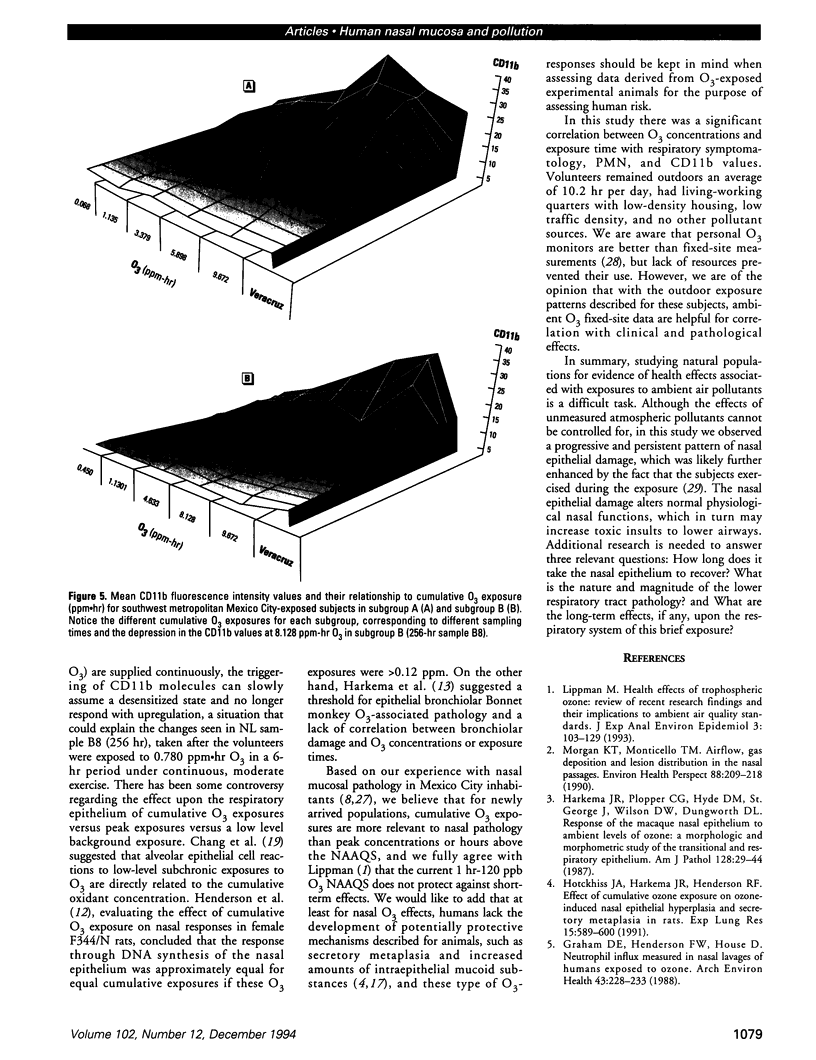
Millions of people worldwide are living in areas where ozone (O3) concentrations exceed health standards (an hourly average of 235 micrograms/m3/0.12 ppm, not to be exceeded more than once per year). Ozone induces acute nasal inflammatory responses and significant epithelial lesions in experimental animals and humans. To determine the nasal effects of a 15-day exposure to an urban polluted atmosphere with O3 as the main pollutant, we studied a population of healthy, young males newly arrived to southwest metropolitan Mexico City (SWMMC). The study included 49 non-smoking residents in an unpolluted port, Veracruz City; 14 subjects stayed in the port and served as controls, while 35 subjects traveled to SWMMC and had serial nasal lavages at different times after arriving in SWMMC. Subjects had exposures to ambient O3 an average of 10.2 hr/day, with a total cumulative O3 exposure of 10.644 ppm.hr. Nasal inflammatory responses, polymorphonuclear leukocyte PMN-CD11b surface expression, rhinoscopic changes, and respiratory symptoms were evaluated. Exposed subjects had massive nasal epithelial shedding and significant responses in PMN nasal influx (p < 0.00001) and in PMN-CD11b expression (p < 0.05). Cumulative O3 exposure correlated with respiratory symptoms, PMNs (rs = 0.2374, p < 0.01), and CD11b (rs = 0.3094, p < 0.01); 94% of exposed subjects experienced respiratory symptoms, and 97% left the city with an abnormal nasal mucosa by rhinoscopy. Nasal epithelial changes persisted 2 weeks after the exposed subjects returned to their nonpolluted environment. Exposure to an urban polluted atmosphere induces significant and persistent nasal epithelial alterations in healthy subjects.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Calderon-Garcidueñas L., Osorno-Velazquez A., Bravo-Alvarez H., Delgado-Chavez R., Barrios-Marquez R. Histopathologic changes of the nasal mucosa in southwest Metropolitan Mexico City inhabitants. Am J Pathol. 1992 Jan;140(1):225–232. [PMC free article] [PubMed] [Google Scholar]
- Calderon-Garcidueñas L., Roy-Ocotla G. Nasal cytology in southwest metropolitan Mexico City inhabitants: a pilot intervention study. Environ Health Perspect. 1993 Jun;101(2):138–144. doi: 10.1289/ehp.101-1519752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang L., Miller F. J., Ultman J., Huang Y., Stockstill B. L., Grose E., Graham J. A., Ospital J. J., Crapo J. D. Alveolar epithelial cell injuries by subchronic exposure to low concentrations of ozone correlate with cumulative exposure. Toxicol Appl Pharmacol. 1991 Jun 15;109(2):219–234. doi: 10.1016/0041-008x(91)90170-j. [DOI] [PubMed] [Google Scholar]
- Fleming J. C., Pahl H. L., Gonzalez D. A., Smith T. F., Tenen D. G. Structural analysis of the CD11b gene and phylogenetic analysis of the alpha-integrin gene family demonstrate remarkable conservation of genomic organization and suggest early diversification during evolution. J Immunol. 1993 Jan 15;150(2):480–490. [PubMed] [Google Scholar]
- Graham D. E., Koren H. S. Biomarkers of inflammation in ozone-exposed humans. Comparison of the nasal and bronchoalveolar lavage. Am Rev Respir Dis. 1990 Jul;142(1):152–156. doi: 10.1164/ajrccm/142.1.152. [DOI] [PubMed] [Google Scholar]
- Graham D., Henderson F., House D. Neutrophil influx measured in nasal lavages of humans exposed to ozone. Arch Environ Health. 1988 May-Jun;43(3):228–233. doi: 10.1080/00039896.1988.9934938. [DOI] [PubMed] [Google Scholar]
- Griffin J. D., Spertini O., Ernst T. J., Belvin M. P., Levine H. B., Kanakura Y., Tedder T. F. Granulocyte-macrophage colony-stimulating factor and other cytokines regulate surface expression of the leukocyte adhesion molecule-1 on human neutrophils, monocytes, and their precursors. J Immunol. 1990 Jul 15;145(2):576–584. [PubMed] [Google Scholar]
- Harkema J. R., Hotchkiss J. A., Henderson R. F. Effects of 0.12 and 0.80 ppm ozone on rat nasal and nasopharyngeal epithelial mucosubstances: quantitative histochemistry. Toxicol Pathol. 1989;17(3):525–535. doi: 10.1177/019262338901700307. [DOI] [PubMed] [Google Scholar]
- Harkema J. R., Plopper C. G., Hyde D. M., St George J. A., Wilson D. W., Dungworth D. L. Response of macaque bronchiolar epithelium to ambient concentrations of ozone. Am J Pathol. 1993 Sep;143(3):857–866. [PMC free article] [PubMed] [Google Scholar]
- Harkema J. R., Plopper C. G., Hyde D. M., St George J. A., Wilson D. W., Dungworth D. L. Response of the macaque nasal epithelium to ambient levels of ozone. A morphologic and morphometric study of the transitional and respiratory epithelium. Am J Pathol. 1987 Jul;128(1):29–44. [PMC free article] [PubMed] [Google Scholar]
- Henderson R. F., Hotchkiss J. A., Chang I. Y., Scott B. R., Harkema J. R. Effect of cumulative exposure on nasal response to ozone. Toxicol Appl Pharmacol. 1993 Mar;119(1):59–65. doi: 10.1006/taap.1993.1044. [DOI] [PubMed] [Google Scholar]
- Hotchkiss J. A., Harkema J. R., Henderson R. F. Effect of cumulative ozone exposure on ozone-induced nasal epithelial hyperplasia and secretory metaplasia in rats. Exp Lung Res. 1991 May-Jun;17(3):589–600. doi: 10.3109/01902149109062867. [DOI] [PubMed] [Google Scholar]
- Liu L. J., Koutrakis P., Suh H. H., Mulik J. D., Burton R. M. Use of personal measurements for ozone exposure assessment: a pilot study. Environ Health Perspect. 1993 Sep;101(4):318–324. doi: 10.1289/ehp.93101318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luscinskas F. W., Cybulsky M. I., Kiely J. M., Peckins C. S., Davis V. M., Gimbrone M. A., Jr Cytokine-activated human endothelial monolayers support enhanced neutrophil transmigration via a mechanism involving both endothelial-leukocyte adhesion molecule-1 and intercellular adhesion molecule-1. J Immunol. 1991 Mar 1;146(5):1617–1625. [PubMed] [Google Scholar]
- Madden M. C., Friedman M., Hanley N., Siegler E., Quay J., Becker S., Devlin R., Koren H. S. Chemical nature and immunotoxicological properties of arachidonic acid degradation products formed by exposure to ozone. Environ Health Perspect. 1993 Jun;101(2):154–164. doi: 10.1289/ehp.93101154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mautz W. J., McClure T. R., Reischl P., Phalen R. F., Crocker T. T. Enhancement of ozone-induced lung injury by exercise. J Toxicol Environ Health. 1985;16(6):841–854. doi: 10.1080/15287398509530792. [DOI] [PubMed] [Google Scholar]
- McKinnon K. P., Madden M. C., Noah T. L., Devlin R. B. In vitro ozone exposure increases release of arachidonic acid products from a human bronchial epithelial cell line. Toxicol Appl Pharmacol. 1993 Feb;118(2):215–223. doi: 10.1006/taap.1993.1027. [DOI] [PubMed] [Google Scholar]
- Moffatt R. K., Hyde D. M., Plopper C. G., Tyler W. S., Putney L. F. Ozone-induced adaptive and reactive cellular changes in respiratory bronchioles of bonnet monkeys. Exp Lung Res. 1987;12(1):57–74. doi: 10.3109/01902148709068814. [DOI] [PubMed] [Google Scholar]
- Morgan K. T., Monticello T. M. Airflow, gas deposition, and lesion distribution in the nasal passages. Environ Health Perspect. 1990 Apr;85:209–218. doi: 10.1289/ehp.85-1568327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plopper C. G., Chow C. K., Dungworth D. L., Tyler W. S. Pulmonary alterations in rats exposed to 0.2 and 0.1 ppm ozone: a correlated morphological and biochemical study. Arch Environ Health. 1979 Nov-Dec;34(6):390–395. doi: 10.1080/00039896.1979.10667438. [DOI] [PubMed] [Google Scholar]
- Schwartz L. W., Dungworth D. L., Mustafa M. G., Tarkington B. K., Tyler W. S. Pulmonary responses of rats to ambient levels of ozone: effects of 7-day intermittent or continuous exposure. Lab Invest. 1976 Jun;34(6):565–578. [PubMed] [Google Scholar]
- Shappell S. B., Toman C., Anderson D. C., Taylor A. A., Entman M. L., Smith C. W. Mac-1 (CD11b/CD18) mediates adherence-dependent hydrogen peroxide production by human and canine neutrophils. J Immunol. 1990 Apr 1;144(7):2702–2711. [PubMed] [Google Scholar]
- Socinski M. A., Cannistra S. A., Sullivan R., Elias A., Antman K., Schnipper L., Griffin J. D. Granulocyte-macrophage colony-stimulating factor induces the expression of the CD11b surface adhesion molecule on human granulocytes in vivo. Blood. 1988 Aug;72(2):691–697. [PubMed] [Google Scholar]
- Tepper J. S., Costa D. L., Lehmann J. R., Weber M. F., Hatch G. E. Unattenuated structural and biochemical alterations in the rat lung during functional adaptation to ozone. Am Rev Respir Dis. 1989 Aug;140(2):493–501. doi: 10.1164/ajrccm/140.2.493. [DOI] [PubMed] [Google Scholar]