Abstract
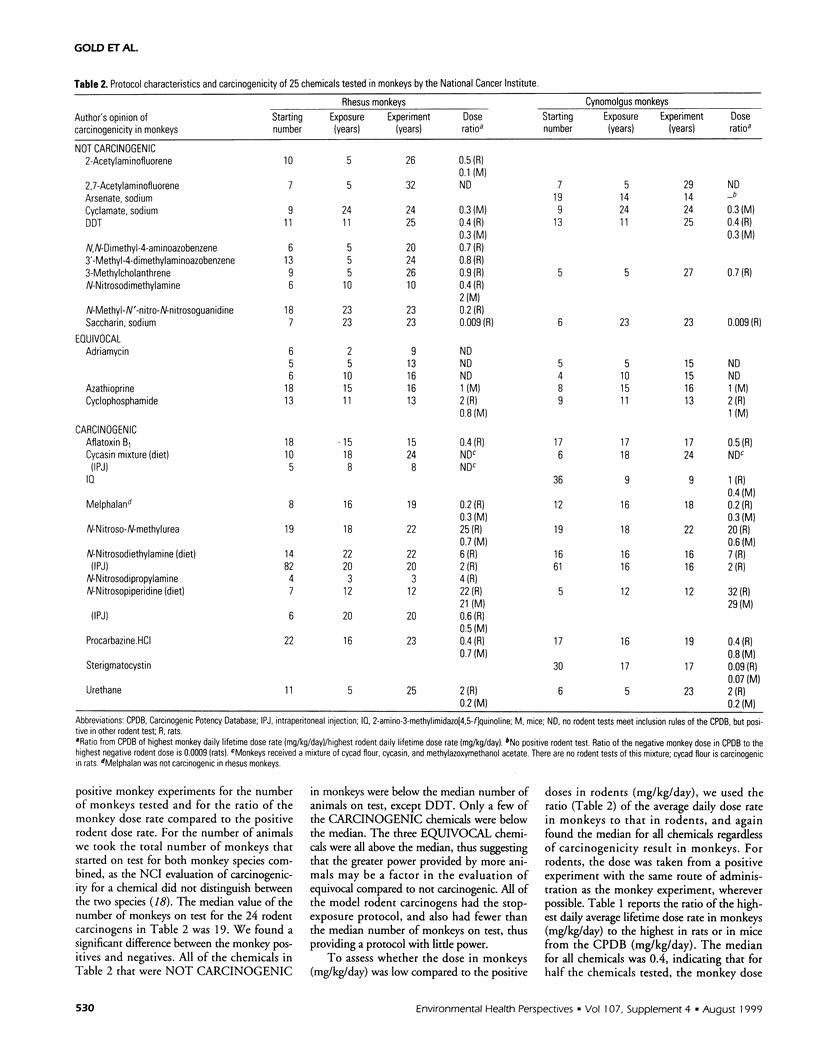
The Carcinogenic Potency Database (CPDB) is a systematic and unifying analysis of results of chronic, long-term cancer tests. This paper presents a supplemental plot of the CPDB, including 513 experiments on 157 test compounds published in the general literature in 1993 and 1994 and in Technical Reports of the National Toxicology Program in 1995 and 1996. The plot standardizes the experimental results (whether positive or negative for carcinogenicity), including qualitative data on strain, sex, route of compound administration, target organ, histopathology, and author's opinion and reference to the published paper, as well as quantitative data on carcinogenic potency, statistical significance, tumor incidence, dose-response curve shape, length of experiment, duration of dosing, and dose rate. A numerical description of carcinogenic potency, the TD(subscript)50(/subscript), is estimated for each set of tumor incidence data reported. When added to the data published earlier, the CPDB now includes results of 5,620 experiments on 1,372 chemicals that have been reported in 1,250 published papers and 414 National Cancer Institute/National Toxicology Program Technical Reports. The plot presented here includes detailed analyses of 25 chemicals tested in monkeys for up to 32 years by the National Cancer Institute. Half the rodent carcinogens that were tested in monkeys were not carcinogenic, despite usually strong evidence of carcinogenicity in rodents and/or humans. Our analysis of possible explanatory factors indicates that this result is due in part to the fact that the monkey studies lacked power to detect an effect compared to standard rodent bioassays. Factors that contributed to the lack of power are the small number of animals on test; a stop-exposure protocol for model rodent carcinogens; in a few cases, toxic doses that resulted in stoppage of dosing or termination of the experiment; and in a few cases, low doses administered to monkeys or early termination of the experiment even though the doses were not toxic. Among chemicals carcinogenic in both monkeys and rodents, there is some support for target site concordance, but it is primarily restricted to liver tumors. Potency values are highly correlated between rodents and monkeys. The plot in this paper can be used in conjunction with the earlier results published in the CRC Handbook of Carcinogenic Potency and Genotoxicity Databases [Gold LS, Zeiger E, eds. Boca Raton FL:CRC Press, 1997] and with our web site (http://potency.berkeley.edu), which includes a guide to the plot of the database, a complete description of the numerical index of carcinogenic potency (TD50), and a discussion of the sources of data, the rationale for the inclusion of particular experiments and particular target sites, and the conventions adopted in summarizing the literature. Two summary tables permit easy access to the literature of animal cancer tests by target organ and by chemical. For readers using the CPDB extensively, a combined plot on diskette or other format is available from the first author. It includes all results published earlier and in this paper, ordered alphabetically by chemical. A SAS database is also available.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ames B. N., Gold L. S. Environmental pollution, pesticides, and the prevention of cancer: misconceptions. FASEB J. 1997 Nov;11(13):1041–1052. doi: 10.1096/fasebj.11.13.9367339. [DOI] [PubMed] [Google Scholar]
- Ames B. N., Shigenaga M. K., Gold L. S. DNA lesions, inducible DNA repair, and cell division: three key factors in mutagenesis and carcinogenesis. Environ Health Perspect. 1993 Dec;101 (Suppl 5):35–44. doi: 10.1289/ehp.93101s535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernstein L., Gold L. S., Ames B. N., Pike M. C., Hoel D. G. Some tautologous aspects of the comparison of carcinogenic potency in rats and mice. Fundam Appl Toxicol. 1985 Feb;5(1):79–86. doi: 10.1016/0272-0590(85)90051-x. [DOI] [PubMed] [Google Scholar]
- Cohen S. M. Role of urinary physiology and chemistry in bladder carcinogenesis. Food Chem Toxicol. 1995 Sep;33(9):715–730. doi: 10.1016/0278-6915(95)00040-9. [DOI] [PubMed] [Google Scholar]
- Freedman D. A., Gold L. S., Slone T. H. How tautological are interspecies correlations of carcinogenic potencies? Risk Anal. 1993 Jun;13(3):265–272. doi: 10.1111/j.1539-6924.1993.tb01078.x. [DOI] [PubMed] [Google Scholar]
- Gold L. S., Manley N. B., Slone T. H., Garfinkel G. B., Ames B. N., Rohrbach L., Stern B. R., Chow K. Sixth plot of the carcinogenic potency database: results of animal bioassays published in the General Literature 1989 to 1990 and by the National Toxicology Program 1990 to 1993. Environ Health Perspect. 1995 Nov;103 (Suppl 8):3–122. doi: 10.1289/ehp.95103s83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold L. S., Manley N. B., Slone T. H., Garfinkel G. B., Rohrbach L., Ames B. N. The fifth plot of the Carcinogenic Potency Database: results of animal bioassays published in the general literature through 1988 and by the National Toxicology Program through 1989. Environ Health Perspect. 1993 Apr;100:65–168. doi: 10.1289/ehp.9310065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold L. S., Sawyer C. B., Magaw R., Backman G. M., de Veciana M., Levinson R., Hooper N. K., Havender W. R., Bernstein L., Peto R. A carcinogenic potency database of the standardized results of animal bioassays. Environ Health Perspect. 1984 Dec;58:9–319. doi: 10.1289/ehp.84589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold L. S., Slone T. H., Ames B. N. What do animal cancer tests tell us about human cancer risk?: Overview of analyses of the carcinogenic potency database. Drug Metab Rev. 1998 May;30(2):359–404. doi: 10.3109/03602539808996318. [DOI] [PubMed] [Google Scholar]
- Gold L. S., Slone T. H., Backman G. M., Eisenberg S., Da Costa M., Wong M., Manley N. B., Rohrbach L., Ames B. N. Third chronological supplement to the carcinogenic potency database: standardized results of animal bioassays published through December 1986 and by the National Toxicology Program through June 1987. Environ Health Perspect. 1990 Mar;84:215–286. doi: 10.1289/ehp.9084215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold L. S., Slone T. H., Backman G. M., Magaw R., Da Costa M., Lopipero P., Blumenthal M., Ames B. N. Second chronological supplement to the Carcinogenic Potency Database: standardized results of animal bioassays published through December 1984 and by the National Toxicology Program through May 1986. Environ Health Perspect. 1987 Oct;74:237–329. doi: 10.1289/ehp.8774237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold L. S., de Veciana M., Backman G. M., Magaw R., Lopipero P., Smith M., Blumenthal M., Levinson R., Bernstein L., Ames B. N. Chronological supplement to the Carcinogenic Potency Database: standardized results of animal bioassays published through December 1982. Environ Health Perspect. 1986 Aug;67:161–200. doi: 10.1289/ehp.8667161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peto R., Pike M. C., Bernstein L., Gold L. S., Ames B. N. The TD50: a proposed general convention for the numerical description of the carcinogenic potency of chemicals in chronic-exposure animal experiments. Environ Health Perspect. 1984 Dec;58:1–8. doi: 10.1289/ehp.84581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharashidze L. K., Beniashvili D. Sh, Sherenesheva N. I., Turkia N. G. Induction of gastric cancer in monkeys by N-methyl-N-nitro-N-nitrosoguanidine (MNNG). Neoplasma. 1989;36(2):129–133. [PubMed] [Google Scholar]
- Tabor E., Kobayashi K. Hepatitis C virus, a causative infectious agent of non-A, non-B hepatitis: prevalence and structure--summary of a conference on hepatitis C virus as a cause of hepatocellular carcinoma. J Natl Cancer Inst. 1992 Jan 15;84(2):86–90. doi: 10.1093/jnci/84.2.86. [DOI] [PubMed] [Google Scholar]
- Thorgeirsson U. P., Dalgard D. W., Reeves J., Adamson R. H. Tumor incidence in a chemical carcinogenesis study of nonhuman primates. Regul Toxicol Pharmacol. 1994 Apr;19(2):130–151. doi: 10.1006/rtph.1994.1013. [DOI] [PubMed] [Google Scholar]


