Abstract
Cell death is a prominent feature of the developing vertebrate nervous system, affecting neurons, glial cells and their progenitors. The most extensively studied and best understood phase of cell death occurs in populations of neurons shortly after they begin establishing connections with other neurons and/or non-neural tissues. This phase of cell death makes appropriate adjustments to the relative sizes of interconnected groups of neurons and matches the size of neuronal populations that innervate non-neural tissues to the optimal requirements of these tissues. The fate of neurons during this period of development is regulated by a variety of secreted proteins that either promote survival or bring about cell death after binding to receptors expressed on the neurons. These proteins may be derived from the targets the neurons innervate, the afferents they receive or from associated glial cells, or they may be secreted by the neurons themselves. In this review, I will outline the established and emerging principles that modulate neuronal number in the developing nervous system.
Keywords: cell death/neurons/neurotrophic factors/survival
Introduction
Cell death plays a key role in regulating the number of neurons in the nervous system from the earliest stages of its development. Whilst cell death has been described in proliferating neuroblasts and in early neurons before they have begun to establish connections with other neurons (de la Rosa and de Pablo, 2000), a prominent phase of cell death occurs in many populations of neurons shortly after their axons reach their targets (Oppenheim, 1991). During this phase of development, 20–80% of neurons with long projecting axons undergo apoptosis and are swiftly removed. Because it is relatively easy to quantify the extent of cell death in anatomically discrete populations of post-mitotic neurons in vivo and to study the survival requirements of these neurons at the corresponding stage in vitro, a good deal has been learnt about the extracellular signals that regulate the survival and death of neurons during this period of development, which will be the primary focus of this review.
The demonstration in a series of classic studies in the chicken embyro that altering target field size prior to innervation affects the number of innervating neurons that survive led to the idea that neuronal death matches the number of neurons to the size and requirements of their target fields (Oppenheim, 1991). A long established idea, the neurotrophic hypothesis, provides an explanation for how target fields influence the size of the neuronal populations that innervate them. This hypothesis arose from work on nerve growth factor (NGF), the first neuron survival factor to be identified. The principal tenet of this hypothesis is that the survival of developing neurons depends on the supply of a neurotrophic factor that is synthesized in limiting amounts in their target fields. The most important evidence for this hypothesis was the demonstration that populations of developing neurons that are supported by NGF in vitro, namely sympathetic neurons and certain kinds of sensory neurons, also depend on NGF in vivo. Anti-NGF antibodies administered during the phase of target field innervation eliminate these neurons, whereas exogenous NGF rescues neurons that would otherwise die (Levi-Montalcini, 1987). These neurons are also lost in mice that lack either NGF or its receptor tyrosine kinase, TrkA (Lewin and Barde, 1996). NGF is synthesized in the target fields of these neurons in proportion to their innervation density during the early stages of their innervation (Harper and Davies, 1990), and is transported retrogradely from the target field in signalling endosomes containing activated TrkA to the neuron cell bodies (Howe et al., 2001).
The discovery of the NGF family of structurally related neurotrophic factors, the neurotrophins, together with studies of the biology of these proteins provided several further examples of the regulation of neuronal survival by retrogradely transported, target-derived neurotrophic factors. In addition to the neurotrophins [NGF, brain-derived neurotrophic factor (BDNF), neurotrophin-3 (NT3) and NT4], several other families of proteins have been shown to promote the survival of various populations of neurons during development. These include the glial cell-derived neurotrophic factor (GDNF) family (GDNF, neurturin, artemin and persephin), the neurotrophic cytokines [ciliary neurotrophic factor (CNTF), leukaemia inhibitory factor (LIF), oncostatin-M (OSM), cardiotrophin-1 (CT-1) and interleukin-6 (IL-6)] and two related factors, hepatocyte growth factor (HGF) and macrophage-stimulating protein (MSP) (Table I). Each of these proteins promotes the survival of various kinds of neurons during particular stages of their development (Davies, 1994b; Lewin and Barde, 1996; Maina and Klein, 1999; Airaksinen and Saarma, 2002). However, studies of the expression of these proteins and their receptors together with studies of their actions in a variety of developing neuronal systems have revealed that neurotrophic factors regulate survival by a variety of routes in addition to the classic target-derived one (Figure 1). A surprising finding in recent years has been the demonstration that certain neurotrophic factors can also promote neuronal death. In addition, several members of the tumour necrosis factor (TNF) superfamily of ligands activate cell death mechanisms in developing neurons and play a role in regulating neuron number during development. In this review, I will give an overview of the interplay of survival-promoting and death-promoting extracellular signals in the regulation of neuronal survival and explain how trophic interactions between different populations of neurons and other tissues are orchestrated by such signals at different stages of development.
Table I. Families of structurally related neurotrophic factors and their receptors.
| Neurotrophic factor family | Neurotrophic factor | Preferred receptor(s) |
|---|---|---|
| Neurotrophins | Nerve growth factor (NGF) | TrkA, p75NTR |
| Brain-derived neurotrophic factor (BDNF) | TrkB, p75NTR | |
| Neurotropin-3 (NT3) | TrkC, p75NTR | |
| Neurotrophin-4 (NT4) | TrkB, p75NTR | |
| GDNF family | Glial cell-derived neurotrophic factor (GDNF) | Ret, GFRα-1 |
| Neurturin | Ret, GFRα-2 | |
| Artemin | Ret, GFRα-3 | |
| Persephin | Ret, GFRα-4 | |
| Neurotrophic cytokines | Ciliary neurotrophic factor (CNTF) | gp130, LIFRβ, CNTFRα |
| Leukaemia-inhibitory factor (LIF) | gp130, LIFRβ | |
| Cardiotrophin-1 (CT-1) | gp130, LIFRβ | |
| Oncostatin-M (OSM) | gp130, OSMRβ | |
| Interleukin-6 (IL-6) | gp130, IL6Rα | |
| HGF family | Hepatocyte growth factor (HGF) | Met |
| Macrophage-stimulating protein (MSP) | Ron |
Although several neurotrophic factors bind and activate more than one member of a family of receptors (e.g. NT3 activates TrkA and TrkB in addition to its preferred receptor tyrosine kinase TrkC), for simplicity, only the preferred receptors for each factor are listed.
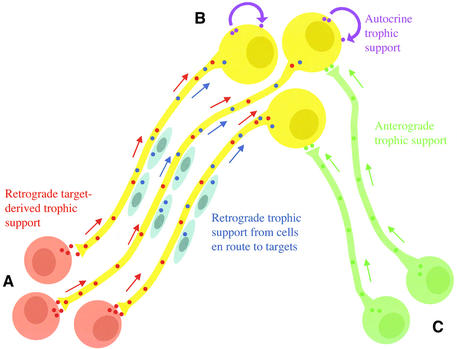
Fig. 1. Schematic illustration of the routes by which neurotrophic factors influence neuronal survival. Three groups of interconnected neurons, A, B and C, are shown. Group B neurons obtain neurotrophic factors from four sources: (i) from target cells or neurons [group A neurons secrete this neurotrophic factor (red dots) which binds to receptors on the axon terminals of neurons B, and is internalized and retrogradely transported (direction of red arrows) to the cell bodies of group A neurons]; (ii) from intermediate target cells or glial cells associated with the axons of neurons B (this neurotrophic factor is represented by the blue dots and is retrogradely transported in the direction of the blue arrows); (iii) from afferents [this neurotrophic factor (green dots) is synthesized in the cell bodies of group C neurons and is anterogradely transported along the axons of these neurons (direction of green arrows) to be released from their terminals and bind to receptors on the cell bodies or dendrites of group B neurons]; and (iv) via an autocrine route (this neurotrophic factor is represented by the purple dots).
In addition to regulating neuronal survival and death, all neurotrophic factors have a wide variety of functions in the nervous system and other tissues (Lewin and Barde, 1996; Maina and Klein, 1999; Bibel and Barde, 2001; Airaksinen and Saarma, 2002) that are beyond the scope of this review, which will focus on the role of these proteins in regulating neuronal survival. I will deal exclusively with extracellular signalling, and will not address intracellular signal transduction events that sustain survival or promote death following receptor binding and activation, as these events are covered by many excellent recent reviews (Miller and Kaplan, 2001; Airaksinen and Saarma, 2002; Hempstead, 2002). I will choose examples from well characterized experimental systems, especially in the peripheral nervous system (PNS), and will focus mostly on neurotrophins. My goal is to illustrate the biological principles and not to provide an exhaustive account.
Retrograde trophic support from innervation targets
The dependence of neurons on a supply of retrogradely transported neurotrophic factor(s) from their target fields raises issues of when and how this dependence is acquired during development. In vitro studies of several populations of PNS neurons from the earliest stages of development suggest that many neurons initially survive independently of neurotrophic factors at the stage when their axons start growing to their targets (Davies, 1994a). Evidence that the duration of neurotrophic factor independence in some neurons is correlated with the time it takes axons to grow to their targets has come from studying populations of cranial sensory neurons whose axons have markedly different distances to grow to their targets (Davies, 1989; Vogel and Davies, 1991). The neurons of the vestibular, geniculate, petrosal and nodose ganglia are derived from thickened regions of head ectoderm termed neurogenic placodes and are born over the same period of development, but differ in the distances their axons have to grow to reach their targets. Vestibular neurons have the closest targets and survive without neurotrophins for only a short time before becoming dependent on BDNF for survival. Nodose neurons have the most distant targets and survive for the longest time before becoming BDNF dependent. Geniculate and petrosal neurons have intermediate target distances and survive for intermediate times before becoming BDNF dependent. The acquisition of BDNF responsiveness is correlated with the expression of the BDNF receptor tyrosine kinase TrkB (Robinson et al., 1996a), and studies of the survival of neurons that differentiate from their progenitor cells in vitro suggest that an intrinsic timing programme specified in the progenitors controls the duration of neurotrophin independence and onset of BDNF receptor expression and dependence (Vogel and Davies, 1991, 1993).
In contrast to the straightforward case of target-derived trophic support synchronized with innervation described above, some neurons have more complex and changing requirements for retrograde trophic support during development. The first and best documented example of neurons that switch their survival requirements from one neurotrophic factor to another during development comes from the neurons of the mouse trigeminal ganglion, a population of cutaneous sensory neurons that innervate the anterior part of the head. If these neurons are cultured at the stage when the earliest neurons are extending axons to their targets, most survive with BDNF or NT3 and very few survive with NGF. In cultures established over the next few days of development, almost all neurons become dependent on NGF and few remain responsive to BDNF or NT3 (Buchman and Davies, 1993). The early survival response to BDNF and NT3 is mediated predominantly via TrkB (Piñón et al., 1996; Davies, 1997). Accordingly, apoptosis is markedly increased in the trigeminal ganglia of trkB–/– embryos and nt3–/– embryos at the stage when the neurons are responsive to BDNF and NT-3 in vitro, and is markedly elevated in trkA–/– embryos later in development when neurons are responsive to NGF (Piñón et al., 1996; Wilkinson et al., 1996). Although this switch in responsiveness is due in part to the sequential generation of BDNF-responsive and NGF-responsive neurons in the ganglion (Enokido et al., 1999; Huang et al., 1999a), bromodeoxyuridine (BrdU) labelling studies have demonstrated that many of the neurons that initially survive with BDNF subsequently switch to become NGF responsive (Enokido et al., 1999). Later in development, toward the end of the phase of naturally occurring neuronal death, trigeminal neurons acquire a survival response to MSP, and by birth the majority of neurons can be supported by either NGF or MSP in culture (Forgie et al., 2003). Accordingly, a significant number of neurons are lost in the trigeminal ganglia of postnatal mice lacking the MSP receptor tyrosine kinase Ron (Forgie et al., 2003). MSP, like NGF, is expressed in the peripheral target field of trigeminal neurons but, whereas NGF expression begins with the arrival of the earliest axons (Davies et al., 1987), MSP expression occurs later in development in accordance with the later response of the neurons to this factor (Forgie et al., 2003). Taken together, these findings suggest that many neurons in the trigeminal ganglion are sequentially dependent in vivo on TrkB, TrkA and Ron signalling during successive developmental stages.
The timing of BDNF/NT3, NGF and MSP responsiveness is correlated with the sequential expression of TrkB, TrkA and Ron receptors on trigeminal neurons (Buchman and Davies, 1993; Ninkina et al., 1996; Forgie et al., 2003). Unlike the onset of BDNF responsiveness in placode-derived sensory neurons, which is largely controlled by an intrinsic timing programme in the neurons, the switch from BDNF/NT3 to NGF dependence appears to be due in part to signals that act on the neurons during the switchover period in vivo (Paul and Davies, 1995; Enokido et al., 1999). Although several studies have shown that NGF increases TrkA expression in neurons and cell lines, the finding that the increase in TrkA mRNA expression that accompanies the onset of a sustained NGF survival in developing trigeminal neurons is unaffected in ngf–/– embryos suggests that target-derived NGF is not involved in regulating NGF receptor expression and dependence during this period of development (Davies et al., 1995b). Interestingly, the loss of BDNF responsiveness is not due simply to reduced TrkB expression, but is associated with the expression of kinase-deficient TrkB isoforms that negatively modulate BDNF signalling (Ninkina et al., 1996).
In addition to requiring different neurotrophic factors during sequential stages of development, the demonstration that proprioceptive neurons can be supported in vitro during the phase of target field innervation by different neurotrophic factors from their peripheral and central target fields raised the possibility that multiple neurotrophic factors might act concurrently to regulate neuronal survival (Davies et al., 1986). This idea has since been substantiated by several well documented in vivo studies. For example, administration to postnatal rats of function-blocking antisera to either NGF or NT3 results in extensive loss of sympathetic neurons (Zhou and Rush, 1995; Tafreshi et al., 1998), and markedly elevated neuronal death occurs in the sympathetic ganglia of both ngf–/– and nt3–/– embryos a few days after the neurons begin to innervate their targets where both NGF and NT3 are produced (Wyatt et al., 1997; Zhou et al., 1997; Francis et al., 1999). Although these results could be explained by the presence of separate subsets of NGF-dependent and NT3-dependent neurons in developing sympathetic ganglia, several observations suggest that NGF and NT3 regulate the survival of the same neurons. NGF and NT3 each promote the survival of the great majority of late fetal and postnatal sympathetic neurons in vitro (Davies et al., 1995a) and neuronal death is not significantly greater in mice that lack both NGF and NT3 compared with mice that lack NGF alone (Francis et al., 1999). Furthermore, analysis of the Trk receptor isoforms expressed by sympathetic neurons (Wyatt et al., 1997), in vitro studies of TrkC-deficient neurons (Davies et al., 1995a) and the effects of a mutant NT3 protein that only signals via TrkA (Belliveau et al., 1997) have shown that both NGF and NT3 promote sympathetic neuron survival by acting via the same receptor, TrkA. Taken together, these results suggest that the survival of the majority of sympathetic neurons is regulated by target-derived NGF and NT3 during the period of development when they are establishing connections with their targets.
Retrograde trophic support from cells en route to innervation targets
Whilst there are clear examples of neurons that survive independently of neurotrophic factors when their axons are growing to their targets, there is evidence that some neurons depend upon trophic support provided by cells that lie en route to their final destination (Figure 1). The finding that BDNF and NT3, which sustain the survival of early trigeminal neurons, are initially expressed in the tissue through which trigeminal axons grow to their peripheral targets from the earliest stages of axonal outgrowth, together with the loss of many trigeminal neurons in trkB–/– and nt3–/– embryos during this early stage of development (Buchman and Davies, 1993; Piñón et al., 1996; Wilkinson et al., 1996), raised the possibility that BDNF and/or NT3 may provide intermediate trophic support to some trigeminal neurons when their axons are growing to their targets (Davies, 1997). Likewise, many dorsal root ganglion (DRG) sensory neurons are dependent on NT3 early in development, and NT3 is expressed in tissues through which the peripheral axons of these neurons grow to their targets (Farinas et al., 1996; White et al., 1996; Liebl et al., 1997).
Additional observations that support the notion of intermediate trophic support have come by studying embryos that lack the ErbB3 neuregulin receptor (Riethmacher et al., 1997). These embryos lack Schwann cells and their precursors that associate with sensory and motor axons. Although sensory and motor neurons start to extend axons normally in these embryos, a substantial number of these neurons subsequently die early in their development. Because Schwann cells and their precursors are known to synthesize a variety of factors that are capable of promoting neuronal survival (Mirsky et al., 2002), it is possible that the loss of sensory and motor neurons in ErbB3-deficient embryos results from a lack of trophic support from Schwann cells and/or their precursors. However, because the enhanced neuronal death in erbB3–/– embryos begins ∼2 days later in development than the enhanced neuronal death observed in trkB–/– or nt3–/– (Piñón et al., 1996; Wilkinson et al., 1996; Liebl et al., 1997), it is possible that Schwann cell-derived trophic support may not be required for sustaining the survival of neurons from the very earliest stages of axonal outgrowth.
Evidence for intermediate trophic support in the developing CNS has been reported for spinal commissural neurons, a group of neurons in the dorsal spinal cord whose axons first grow ventrally to the floor plate of the spinal cord where they cross the midline and ascend to targets in the brain (Wang and Tessier-Lavigne, 1999). Floor plate conditioned medium was able to maintain the integrity of commissural axons growing from dorsal spinal cord explants and reduce the number of dying cells in the explants at the stage of development when many of the commissural axons would normally be in the vicinity of the floor plate, suggesting that the floor plate may be a source of trophic support for these neurons as their axons make their way to their final destination. However, without knowing the identity of the putative neurotrophic factor(s) and which cells express receptors for it, it is uncertain whether the well-being of commissural neurons in these explants is mediated by a direct action of the putative floor plate-derived trophic factor on these neurons or indirectly via effects on other cells in the explants.
Anterograde trophic support
The demonstration in several populations of developing neurons that deafferentation increases and hyperinnervation decreases the number of neurons lost during the stage of naturally occurring neuronal death (Linden, 1994) has given rise to the idea that neurons depend in part for their survival on trophic support derived from their afferents (Figure 1). Many neurotrophic factors are synthesized in various neurons, and evidence for anterograde transport of endogenous neurotrophic factors along axons has been demonstrated in several locations. For example, endogenous BDNF is transported along the axons of a subset of sensory neurons in vivo (Zhou and Rush, 1996; Michael et al., 1997), and is released from these neurons in vitro where it can sustain the survival of co-cultured BDNF-dependent neurons (Robinson et al., 1996b). BDNF is widely distributed in nerve terminals in the brain, and inhibition of axonal transport depletes BDNF from terminals (Altar et al., 1997; Conner et al., 1997). Antero grade transport of endogenous NT3 has also been demonstrated along retinal ganglion cell axons (von Bartheld and Butowt, 2000).
Although anterogradely transported BDNF can function as a neurotransmitter (Barde, 2002; Blum et al., 2002) and has a role in regulating dendritic growth (McAllister et al., 1999), several studies have shown that anterogradely transported BDNF can also promote the survival of post-synaptic neurons during development. For example, endogenous BDNF is anterogradely transported along cortical axons to the striatum, and a subset of striatal neurons is lost in BDNF-deficient mice, suggesting that these neurons are supported by BDNF from cortical afferents (Altar et al., 1997). Motoneuron death following axotomy is reduced in mice that overexpress BDNF in noradrenergic neurons that project to motoneurons, suggesting that these noradrenergic neurons are capable of providing anterograde trophic support to the neurons they innervate (Fawcett et al., 1998). Reducing the availability of endogenous, anterogradely transported BDNF within the superior colliculus (a midbrain structure) using function-blocking antibodies in postnatal rats increases neuron death in this structure, suggesting that BDNF released from retinal ganglion cell axons acts as a survival factor for these neurons (Caleo et al., 2000).
Autocrine trophic support
Evidence that neurotrophins can act by an autocrine route (Figure 1) initially came from in vitro studies of early DRG neurons that express BDNF and undergo an early morphological transition shortly after they differentiate from progenitor cells. This morphological transition occurs in single cell cultures, is accelerated by exogenous BDNF and is inhibited by antisense BDNF oligonucleotides (Wright et al., 1992). This BDNF autocrine loop is mediated via the common neurotrophin receptor p75NTR, and is not required for survival during this early stage of neuronal development (Wright et al., 1992; Huber et al., 2000). Similar experiments on adult DRG neurons, most of which survive in culture without added neurotrophic factors, raised the possibility that a BDNF autocrine loop is responsible for sustaining the survival of a subset of these neurons in culture (Acheson et al., 1995). However, in adults, BDNF is expressed mainly by TrkA-expressing, not TrkB-expressing, DRG neurons (Kashiba et al., 1997). Since the survival-promoting effects of BDNF are mediated via TrkB (Lewin and Barde, 1996), it seems unlikely that a BDNF autocrine loop plays a significant role in sustaining the survival of adult DRG neurons in vivo. In the central nervous system (CNS), however, BDNF and TrkB are co-expressed on a variety of neurons in the cerebral cortex, hippocampus and cerebellum (Kokaia et al., 1993; Miranda et al., 1993; Ferrer et al., 1997; Schwartz et al., 1997; Pitts and Miller, 2000; Dieni and Rees, 2002). The demonstration that anti-BDNF antiserum reduces neuronal survival in cortical neuron cultures is consistent with the operation of a BDNF autocrine loop in some cortical neurons (Ghosh et al., 1994). The finding that horizontal neurons in the developing retina co-express TrkA and NGF and that these neurons are killed by antisense NGF oligonucleotides suggests that their survival is sustained by an NGF autocrine loop (Karlsson et al., 2001).
In addition to the BDNF autocrine loop in differentiating DRG neurons (Wright et al., 1992), there is evidence for other neurotrophic factor autocrine loops in differentiating neurons elsewhere in the nervous system. In vivo and in vitro studies have implicated an HGF autocrine loop in sustaining the survival of sympathetic neuroblasts. These cells express HGF and its receptor tyrosine kinase Met and survive for >2 days in very low density culture in defined medium lacking neurotrophic factors. Neuroblasts die more rapidly in the presence of anti-HGF, and neuroblasts lacking a functional Met receptor also die more rapidly in vitro and in vivo (Maina et al., 1998). An activity-dependent NT3 autocrine loop has been shown in single-cell cultures to promote the differentiation of a subset of hippocampal pyramidal neurons (Boukhaddaoui et al., 2001).
A fascinating and unexpected development in neurotrophic factor survival signalling is the demonstration that neurotrophic cytokines mediate their survival effects on cultured motoneurons by inducing the expression and secretion of another protein, Reg-2. Purified Reg-2 is a survival factor for motoneurons on its own, and blocking Reg-2 expression using Reg-2 antisense adenovirus abrogates the survival effect of CNTF (Nishimune et al., 2000). These data raise the intriguing possibility that a Reg-2 autocrine loop mediates the survival effect of CNTF on motoneurons.
Cytotoxic actions of neurotrophins and other factors on developing neurons
An interesting and surprising development in the neurotrophic field in recent years is the demonstration that neurotrophins can promote cell death under certain circumstances. Neurotrophins not only bind and activate their cognate Trk receptor tyrosine kinases, but also bind the common neurotrophin receptor p75NTR. This receptor has a multitude of functions. In addition to neurotrophins, it binds several other proteins with nanomolar affinities, including the neurotoxic prion protein fragment PrP and the Aβ amyloid peptide, and is a co-receptor for myelin-derived axon growth inhibitory proteins (Dechant and Barde, 2002; Hempstead, 2002). p75NTR is a member of the TNF receptor superfamily and, like several other members of this superfamily of receptors, possesses an intracellular death domain which is responsible for assembling a death-inducing signalling complex, leading to caspase activation and cell death (Locksley et al., 2001). However, the influence of p75NTR on cell survival and death in response to neurotrophins depends in part upon the cellular context within which it acts. In TrkA-expressing neurons, the presence of p75NTR enhances the survival-promoting action of NGF (Davies et al., 1993; Horton et al., 1997; Ryden et al., 1997). This is due to a p75NTR-induced conformational change in TrkA that increases its affinity for NGF (Esposito et al., 2001) and to survival signalling cascades initiated by p75NTR itself (Hamanoue et al., 1999; DeFreitas et al., 2001; Roux et al., 2001). Under conditions of reduced or absent Trk signalling, p75NTR can promote cell death (Barrett and Bartlett, 1994; Casaccia-Bonnefil et al., 1996; Frade et al., 1996; Bamji et al., 1998; Davey and Davies, 1998). The physiological relevance of p75NTR in promoting cell death has been demonstrated in vivo by showing that basal forebrain cholinergic neurons, which normally express high levels of p75NTR, are present in elevated numbers in postnatal mice that lack p75NTR (Van der Zee et al., 1996; Naumann et al., 2002).
The effect of NGF on cell survival also depends on whether it acts in either its processed or unprocessed form. The neurotrophins, in common with many other growth factors, are synthesized as larger precursor proteins that are proteolytically cleaved to generate the mature ligands. ProNGF has a >5-fold greater affinity for p75NTR than mature NGF and has negligible binding to TrkA. As such, proNGF is a much more potent inducer of cell death than mature NGF (Lee et al., 2001). Since both proNGF and proBDNF are relatively abundant in brain (Fahnestock et al., 2001; Mowla et al., 2001), it is possible that the synthesis and location of specific proteases involved in post-secretory processing of neurotrophin precursor proteins could play a role in regulating neurotrophin function (Ibanez, 2002).
Several other members of the TNF receptor superfamily have been implicated in regulating neuronal death in the developing nervous system. Blocking the interaction between Fas and its ligand (Fas-L) reduces cell death induced by neurotrophic factor deprivation in cerebellar granule cells (Brunet et al., 1999) and spinal motoneurons (Raoul et al., 1999). Fas is constitutively expressed in these cells, and Fas-L is induced following neurotrophic factor deprivation. These results suggest that Fas signalling is triggered by neurotrophic factor withdrawal, and this in turn brings about the demise of the neurons (Raoul et al., 1999). These intriguing results raise the possiblity that Fas signalling plays a role promoting apoptosis during the period of naturally occurring neuronal death in vivo, although additional work on embryos defective in Fas signalling will be required to confirm this possibility.
TNF-α has also been implicated in promoting the death of sympathetic and sensory neurons during the phase of naturally occurring neuronal death (Barker et al., 2001). Function-blocking antibodies to either TNF-α or its death domain-possessing receptor TNFRI rescue many of these neurons following NGF deprivation in vitro, and fewer sensory and sympathetic neurons die during the phase of naturally occurring neuronal death in TNF-α-deficient embryos in vivo. Because the neurons co-express TNF-α and TNFRI, it is likely that TNF-α acts by an autocrine loop to facilitate the death of neurons that fail to procure an adequate supply of NGF. This TNF-α autocrine loop is not an obligatory step in the apoptosis of neurotrophin-deprived neurons because wild-type neurons treated with anti-TNF-α or anti-TNFRI antibodies as well as TNF-α-deficient neurons still die in culture following neurotrophin deprivation, but do so more slowly than neurons in which TNF-α signalling is intact (Barker et al., 2001). Thus, it is likely that the TNF-α autocrine loop accelerates the death of neurons that fail to procure an adequate supply of neurotrophic factor.
Studies of FasL and TNF-α add an unexpected twist to the molecular mechanisms that regulate the death of neurons during development. Neuronal death is not simply due to withdrawal of survival signals, but is due in part to the activation of cytotoxic signalling mechanisms. In future work, it will be important to explore the potential role of other death domain-containing members of the TNF receptor superfamily in regulating neuronal death. In addition to accelerating the demise of neurons that fail to procure sufficient neurotrophic support, it will be interesting to ascertain whether such factors could, in some locations, override trophic signals and selectively eliminate inappropriately connected neurons directly.
Conclusions and future directions
From the pioneering years of neurotrophic factor biology in the middle of the last century to the present time, there has been substantial growth in our understanding of the mechanisms that regulate the relative sizes of interconnected populations of neurons in the developing nervous system. Whilst the neurotrophic hypothesis has become a substantiated cornerstone of our thinking in this field, the shear complexity and subtleties of the cell–cell interactions involved in adjusting neuron number by regulating cell survival and cell death is quite remarkable. A multitude and growing number of extracellular proteins have been implicated in this process. Many of these proteins act in a variety of ways—retrograde, anterograde and autocrine—in different locations and at different stages of development, and some proteins promote survival in one situation and death in another. It seems likely that each population of neurons in the developing nervous system is exposed at different stages of development to a variety of survival-enhancing and death-promoting signals derived from their targets, afferents and in some cases from the neurons themselves.
We are still at the stage of describing and elucidating the basic principles of cell–cell communication mediated by secreted and expressed proteins that influence life and death decisions in developing neurons. How the trophic interactions between interconnected neurons are orchestrated is a fundamentally important but largely mysterious process. For example, the acquisition of survival dependence by some populations of neurons appears to be controlled by an intrinsic timing mechanism independent of target contact, but we have little understanding of how neurons become programmed to express the appropriate receptors at the right time in development. Likewise, the sensitivity of neurons to particular neurotrophic factors is a crucial element that governs how many neurons within a particular population survive, yet we know little about what regulates the level of receptor expression and the other molecular processes that control the sensitivity of developing neurons to particular factors in vivo. Studies of neurotrophin receptor expression in mice with null mutations in transcription factors such as Brn-3a are, however, starting to shed light on this important topic (Huang et al., 1999b). The timing and level of NGF synthesis in some targets is coordinated with the arrival of the earliest nerve fibres and matched to initial innervation density (Davies et al., 1987; Harper and Davies, 1990), yet appears to be regulated independently of innervation (Rohrer et al., 1988). Again, little is known about how the timing, site and level of synthesis of this and other neurotrophic factors are regulated in vivo. However, elegant studies of NT3 expression, which like NGF occurs independently of innervation, have begun to make inroads into this crucial issue by demonstrating in the developing limb that epithelial–mesenchymal interactions mediated by Wnt factors play a key role (Patapoutian et al., 1999). These and many other key issues such as understanding more fully the intracellular signalling mechanisms that transduce, integrate and execute survival and death signals within neurons will provide no shortage of fascination, industry and surprises in this field for many years to come.
Acknowledgments
Acknowledgements
I would like to thank all friends and colleagues for giving me plenty to write about in this review and apologize to those whose work has not been included because of constraints on space or ignorance on my part. My research in this field has been supported by the Wellcome Trust, MRC, BBSRC, CRC, Royal Society, Action Research and the European Commission.
References
- Acheson A., Conover,J.C., Fandl,J.P., DeChlara,T.M., Russell,M., Thadani,A., Squinto,S.P., Yancopoulos,G.D. and Lindsay,R.M. (1995) A BDNF autocrine loop in adult sensory neurons prevents cell death. Nature, 374, 450–453. [DOI] [PubMed] [Google Scholar]
- Airaksinen M.S. and Saarma,M. (2002) The GDNF family: signalling, biological functions and therapeutic value. Nat. Rev. Neurosci., 3, 383–394. [DOI] [PubMed] [Google Scholar]
- Altar C.A., Cai,N., Bliven,T., Juhasz,M., Conner,J.M., Acheson,A.L., Lindsay,R.M. and Wiegand,S.J. (1997) Anterograde transport of brain-derived neurotrophic factor and its role in the brain. Nature, 389, 856–860. [DOI] [PubMed] [Google Scholar]
- Bamji S.X., Majdan,M., Pozniak,C.D., Belliveau,D.J., Aloyz,R., Kohn,J., Causing,C.G. and Miller,F.D. (1998) The p75 neurotrophin receptor mediates neuronal apoptosis and is essential for naturally occurring sympathetic neuronal death. J. Cell Biol., 140, 911–923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barde Y.A. (2002) Neurobiology: neurotrophin channels excitement. Nature, 419, 683–684. [DOI] [PubMed] [Google Scholar]
- Barker V., Middleton,G., Davey,F. and Davies,A.M. (2001) TNFα contributes to the death of NGF-dependent neurons during development. Nat. Neurosci., 4, 1194–1198. [DOI] [PubMed] [Google Scholar]
- Barrett G.L. and Bartlett,P.F. (1994) The p75 nerve growth factor receptor mediates survival or death depending on the stage of sensory neuron development. Proc. Natl Acad. Sci. USA, 91, 6501–6505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belliveau D.J., Krivko,I., Kohn,J., Lachance,C., Pozniak,C., Rusakov,D., Kaplan,D. and Miller,F.D. (1997) NGF and neurotrophin-3 both activate TrkA on sympathetic neurons but differentially regulate survival and neuritogenesis. J. Cell Biol., 136, 375–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bibel M. and Barde,Y.A. (2001) Neurotrophins: key regulators of cell fate and cell shape in the vertebrate nervous system. Genes Dev., 14, 2919–2937. [DOI] [PubMed] [Google Scholar]
- Blum R., Kafitz,K.W. and Konnerth,A. (2002) Neurotrophin-evoked depolarization requires the sodium channel Na(V)1.9. Nature, 419, 687–693. [DOI] [PubMed] [Google Scholar]
- Boukhaddaoui H., Sieso,V., Scamps,F. and Valmier,J. (2001) An activity-dependent neurotrophin-3 autocrine loop regulates the phenotype of developing hippocampal pyramidal neurons before target contact. J. Neurosci., 21, 8789–8797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunet A. et al. (1999) Akt promotes cell survival by phosphorylating and inhibiting a Forkhead transcription factor. Cell, 96, 857–868. [DOI] [PubMed] [Google Scholar]
- Buchman V.L. and Davies,A.M. (1993) Different neurotrophins are expressed and act in a developmental sequence to promote the survival of embryonic sensory neurons. Development, 118, 989–1001. [DOI] [PubMed] [Google Scholar]
- Caleo M., Menna,E., Chierzi,S., Cenni,M.C. and Maffei,L. (2000) Brain-derived neurotrophic factor is an anterograde survival factor in the rat visual system. Curr. Biol., 10, 1155–1161. [DOI] [PubMed] [Google Scholar]
- Casaccia-Bonnefil P., Carter,B.D., Dobrowsky,R.T. and Chao,M.V. (1996) Death of oligodendrocytes mediated by the interaction of nerve growth factor with its receptor p75. Nature, 383, 716–719. [DOI] [PubMed] [Google Scholar]
- Conner J.M., Lauterborn,J.C., Yan,Q., Gall,C.M. and Varon,S. (1997) Distribution of brain-derived neurotrophic factor (BDNF) protein and mRNA in the normal adult rat CNS: evidence for anterograde axonal transport. J. Neurosci., 17, 2295–2313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davey F. and Davies,A.M. (1998) TrkB signalling inhibits p75-mediated apoptosis induced by NGF in embryonic proprioceptive neurons. Curr. Biol., 8, 915–918. [DOI] [PubMed] [Google Scholar]
- Davies A.M. (1989) Intrinsic differences in the growth rate of early nerve fibres related to target distance. Nature, 337, 553–555. [DOI] [PubMed] [Google Scholar]
- Davies A.M. (1994a) Intrinsic programmes of growth and survival in developing vertebrate neurons. Trends Neurosci., 17, 195–199. [DOI] [PubMed] [Google Scholar]
- Davies A.M. (1994b) Role of neurotrophins in the developing nervous system. J. Neurobiol., 25, 1334–1348. [DOI] [PubMed] [Google Scholar]
- Davies A.M. (1997) Neurotrophin switching: where does it stand. Curr. Opin. Neurobiol., 7, 110–118. [DOI] [PubMed] [Google Scholar]
- Davies A.M., Bandtlow,C., Heumann,R., Korsching,S., Rohrer,H. and Thoenen,H. (1987) Timing and site of nerve growth factor synthesis in developing skin in relation to innervation and expression of the receptor. Nature, 326, 353–358. [DOI] [PubMed] [Google Scholar]
- Davies A.M., Thoenen,H. and Barde,Y.A. (1986) Different factors from the central nervous system and periphery regulate the survival of sensory neurones. Nature, 319, 497–499. [DOI] [PubMed] [Google Scholar]
- Davies A.M., Lee,K.F. and Jaenisch,R. (1993) p75-deficient trigeminal sensory neurons have an altered response to NGF but not to other neurotrophins. Neuron, 11, 565–574. [DOI] [PubMed] [Google Scholar]
- Davies A.M., Minichiello,L. and Klein,R. (1995a) Developmental changes in NT3 signalling via TrkA and TrkB in embryonic neurons. EMBO J., 14, 4482–4489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies A.M., Wyatt,S., Nishimura,M. and Phillips,H. (1995b) NGF receptor expression in sensory neurons develops normally in embryos lacking NGF. Dev. Biol., 171, 434–438. [DOI] [PubMed] [Google Scholar]
- de la Rosa E.J. and de Pablo,F. (2000) Cell death in early neural development: beyond the neurotrophic theory. Trends Neurosci., 23, 454–458. [DOI] [PubMed] [Google Scholar]
- Dechant G. and Barde,Y.A. (2002) The neurotrophin receptor p75(NTR): novel functions and implications for diseases of the nervous system. Nat. Neurosci., 5, 1131–1136. [DOI] [PubMed] [Google Scholar]
- DeFreitas M.F., McQuillen,P.S. and Shatz,C.J. (2001) A novel p75NTR signaling pathway promotes survival, not death, of immunopurified neocortical subplate neurons. J. Neurosci., 21, 5121–5129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dieni S. and Rees,S. (2002) Distribution of brain-derived neurotrophic factor and TrkB receptor proteins in the fetal and postnatal hippocampus and cerebellum of the guinea pig. J. Comp. Neurol., 454, 229–240. [DOI] [PubMed] [Google Scholar]
- Enokido Y., Wyatt,S. and Davies,A.M. (1999) Developmental changes in the response of trigeminal neurons to neurotrophins: influence of birthdate and the ganglion environment. Development, 126, 4365–4373. [DOI] [PubMed] [Google Scholar]
- Esposito D., Patel,P., Stephens,R.M., Perez,P., Chao,M.V., Kaplan,D.R. and Hempstead,B.L. (2001) The cytoplasmic and transmembrane domains of the p75 and Trk A receptors regulate high affinity binding to nerve growth factor. J. Biol. Chem., 276, 32687–32695. [DOI] [PubMed] [Google Scholar]
- Fahnestock M., Michalski,B., Xu,B. and Coughlin,M.D. (2001) The precursor pro-nerve growth factor is the predominant form of nerve growth factor in brain and is increased in Alzheimer’s disease. Mol. Cell. Neurosci., 18, 210–220. [DOI] [PubMed] [Google Scholar]
- Farinas I., Yoshida,C.K., Backus,C. and Reichardt,L.F. (1996) Lack of neurotrophin-3 results in death of spinal sensory neurons and premature differentiation of their precursors. Neuron, 17, 1065–1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fawcett J.P., Bamji,S.X., Causing,C.G., Aloyz,R., Ase,A.R., Reader,T.A., McLean,J.H. and Miller,F.D. (1998) Functional evidence that BDNF is an anterograde neuronal trophic factor in the CNS. J. Neurosci., 18, 2808–2821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrer I., Ballabriga,J., Marti,E., Pozas,E., Planas,A.M. and Blasi,J. (1997) BDNF and TrkB co-localize in CA1 neurons resistant to transient forebrain ischemia in the adult gerbil. J. Neuropathol. Exp. Neurol., 56, 790–797. [PubMed] [Google Scholar]
- Forgie A., Wyatt,S., Correll,P. and Davies,A.M. (2003) Macrophage stimulating protein (MSP) is a novel target-derived neurotrophic factor for developing sensory and sympathetic neurons. Development, 130, 995–1002. [DOI] [PubMed] [Google Scholar]
- Frade J.M., Rodriguez-Tebar,A. and Barde,Y.A. (1996) Induction of cell death by endogenous nerve growth factor through its p75 receptor. Nature, 383, 166–168. [DOI] [PubMed] [Google Scholar]
- Francis N., Farinas,I., Brennan,C., Rivas-Plata,K., Backus,C., Reichardt,L. and Landis,S. (1999) NT-3, like NGF, is required for survival of sympathetic neurons, but not their precursors. Dev. Biol., 210, 411–427. [DOI] [PubMed] [Google Scholar]
- Ghosh A., Carnahan,J. and Greenberg,M.E. (1994) Requirement for BDNF in activity-dependent survival of cortical neurons. Science, 263, 1618–1623. [DOI] [PubMed] [Google Scholar]
- Hamanoue M., Middleton,G., Wyatt,S., Jaffrey,E., Hay,R.T. and Davies,A.M. (1999) p75-mediated NF-κB activation enhances the survival response of developing sensory neurons to nerve growth factor. Mol. Cell. Neurosci., 14, 28–40. [DOI] [PubMed] [Google Scholar]
- Harper S. and Davies,A.M. (1990) NGF mRNA expression in developing cutaneous epithelium related to innervation density. Development, 110, 515–519. [DOI] [PubMed] [Google Scholar]
- Hempstead B.L. (2002) The many faces of p75NTR. Curr. Opin. Neurobiol., 12, 260–267. [DOI] [PubMed] [Google Scholar]
- Horton A.R., Laramee,G., Wyatt,S., Shih,A., Winslow,J. and Davies,A.M. (1997) NGF binding to p75 enhances the sensitivity of sensory and sympathetic neurons to NGF at different stages of development. Mol. Cell. Neurosci., 10, 162–172. [DOI] [PubMed] [Google Scholar]
- Howe C.L., Valletta,J.S., Rusnak,A.S. and Mobley,W.C. (2001) NGF signaling from clathrin-coated vesicles: evidence that signaling endosomes serve as a platform for the Ras–MAPK pathway. Neuron, 32, 801–814. [DOI] [PubMed] [Google Scholar]
- Huang E.J., Wilkinson,G.A., Farinas,I., Backus,C., Zang,K., Wong,S.L. and Reichardt,L.F. (1999a) Expression of Trk receptors in the developing mouse trigeminal ganglion: in vivo evidence for NT-3 activation of TrkA and TrkB in addition to TrkC. Development, 126, 2191–2203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang E.J., Zang,K., Schmidt,A., Saulys,A., Xiang,M. and Reichardt,L.F. (1999b) POU domain factor Brn-3a controls the differentiation and survival of trigeminal neurons by regulating Trk receptor expression. Development, 126, 2869–2882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber K., Kuehnel,F., Wyatt,S. and Davies,A.M. (2000) TrkB expression and early sensory neuron survival are independent of endogenous BDNF. J. Neurosci. Res., 59, 372–378. [DOI] [PubMed] [Google Scholar]
- Ibanez C.F. (2002) Jekyll-Hyde neurotrophins: the story of proNGF. Trends Neurosci., 25, 284–286. [DOI] [PubMed] [Google Scholar]
- Karlsson M., Mayordomo,R., Reichardt,L.F., Catsicas,S., Karten,H. and Hallbook,F. (2001) Nerve growth factor is expressed by postmitotic avian retinal horizontal cells and supports their survival during development in an autocrine mode of action. Development, 128, 471–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kashiba H., Ueda,Y., Ueyama,T., Nemoto,K. and Senba,E. (1997) Relationship between BDNF- and trk-expressing neurones in rat dorsal root ganglion: an analysis by in situ hybridization. Neuroreport, 8, 1229–1234. [DOI] [PubMed] [Google Scholar]
- Kokaia Z., Bengzon,J., Metsis,M., Kokaia,M., Persson,H. and Lindvall,O. (1993) Coexpression of neurotrophins and their receptors in neurons of the central nervous system. Proc. Natl Acad. Sci. USA, 90, 6711–6715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee R., Kermani,P., Teng,K.K. and Hempstead,B.L. (2001) Regulation of cell survival by secreted proneurotrophins. Science, 294, 1945–1948. [DOI] [PubMed] [Google Scholar]
- Levi-Montalcini R. (1987) The nerve growth factor 35 years later. Science, 237, 1154–1162. [DOI] [PubMed] [Google Scholar]
- Lewin G.R. and Barde,Y.A. (1996) Physiology of the neurotrophins. Annu. Rev. Neurosci. 19, 289–317. [DOI] [PubMed] [Google Scholar]
- Liebl D.J., Tessarollo,L., Palko,M.E. and Parada,L.F. (1997) Absence of sensory neurons before target innervation in brain-derived neurotrophic factor-, neurotrophin 3- and TrkC-deficient embryonic mice. J. Neurosci., 17, 9113–9121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Locksley R.M., Killeen,N. and Lenardo,M.J. (2001) The TNF and TNF receptor superfamilies: integrating mammalian biology. Cell, 104, 487–501. [DOI] [PubMed] [Google Scholar]
- Linden R. (1994) The survival of developing neurons: a review of afferent control. Neuroscience, 58, 671–682. [DOI] [PubMed] [Google Scholar]
- Maina F. and Klein,R. (1999) Hepatocyte growth factor, a versatile signal for developing neurons. Nat. Neurosci., 2, 213–217. [DOI] [PubMed] [Google Scholar]
- Maina F., Hilton,M.C. andres,R., Wyatt,S., Klein,R. and Davies,A.M. (1998) Multiple roles for hepatocyte growth factor in sympathetic neuron development. Neuron, 20, 835–846. [DOI] [PubMed] [Google Scholar]
- McAllister A.K., Katz,L.C. and Lo,D.C. (1999) Neurotrophins and synaptic plasticity. Annu. Rev. Neurosci., 22, 295–318. [DOI] [PubMed] [Google Scholar]
- Michael G.J., Averill,S., Nitkunan,A., Rattray,M., Bennett,D.L., Yan,Q. and Priestley,J.V. (1997) Nerve growth factor treatment increases brain-derived neurotrophic factor selectively in TrkA-expressing dorsal root ganglion cells and in their central terminations within the spinal cord. J. Neurosci., 17, 8476–8490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller F.D. and Kaplan,D.R. (2001) Neurotrophin signalling pathways regulating neuronal apoptosis. Cell. Mol. Life Sci., 58, 1045–1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miranda R.C., Sohrabji,F. and Toran-Allerand,C.D. (1993) Neuronal co-localisation of mRNAs for neurotrophins and their receptors in the developing central nervous system suggests a potential for autocrine interactions. Proc. Natl Acad. Sci. USA, 90, 6439–6443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirsky R., Jessen,K.R., Brennan,A., Parkinson,D., Dong,Z., Meier,C., Parmantier,E. and Lawson,D. (2002) Schwann cells as regulators of nerve development. J. Physiol. (Paris), 96, 17–24. [DOI] [PubMed] [Google Scholar]
- Mowla S.J., Farhadi,H.F., Pareek,S., Atwal,J.K., Morris,S.J., Seidah,N.G. and Murphy,R.A. (2001) Biosynthesis and post-translational processing of the precursor to brain-derived neurotrophic factor. J. Biol. Chem., 276, 12660–12666. [DOI] [PubMed] [Google Scholar]
- Naumann T., Casademunt,E., Hollerbach,E., Hofmann,J., Dechant,G., Frotscher,M. and Barde,Y.A. (2002) Complete deletion of the neurotrophin receptor p75NTR leads to long-lasting increases in the number of basal forebrain cholinergic neurons. J. Neurosci., 22, 2409–2418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ninkina N., Adu,J., Fischer,A., Pinon,L.G., Buchman,V.L. and Davies,A.M. (1996) Expression and function of TrkB variants in developing sensory neurons. EMBO J., 15, 6385–6393. [PMC free article] [PubMed] [Google Scholar]
- Nishimune H., Vasseur,S., Wiese,S., Birling,M.C., Holtmann,B., Sendtner,M., Iovanna,J.L. and Henderson,C.E. (2000) Reg-2 is a motoneuron neurotrophic factor and a signalling intermediate in the CNTF survival pathway. Nat. Cell Biol., 2, 906–914. [DOI] [PubMed] [Google Scholar]
- Oppenheim R.W. (1991) Cell death during development of the nervous system. Annu. Rev. Neurosci., 14, 453–501. [DOI] [PubMed] [Google Scholar]
- Patapoutian A., Backus,C., Kispert,A. and Reichardt,L.F. (1999) Regulation of neurotrophin-3 expression by epithelial–mesenchymal interactions: the role of Wnt factors. Science, 283, 1180–1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul G. and Davies,A.M. (1995) Trigeminal sensory neurons require extrinsic signals to switch neurotrophin dependence during the early stages of target field innervation. Dev. Biol., 171, 590–605. [DOI] [PubMed] [Google Scholar]
- Piñón L.G.P., Minichiello,L., Klein,R. and Davies,A.M. (1996) Timing of neuronal death in trkA, trkB and trkC mutant embryos reveals developmental changes in sensory neuron dependence on Trk signalling. Development, 122, 3255–3261. [DOI] [PubMed] [Google Scholar]
- Pitts A.F. and Miller,M.W. (2000) Expression of nerve growth factor, brain-derived neurotrophic factor and neurotrophin-3 in the somatosensory cortex of the mature rat: coexpression with high-affinity neurotrophin receptors. J. Comp. Neurol., 418, 241–254. [DOI] [PubMed] [Google Scholar]
- Raoul C., Henderson,C.E. and Pettmann,B. (1999) Programmed cell death of embryonic motoneurons triggered through the Fas death receptor. J. Cell Biol., 147, 1049–1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riethmacher D., Sonnenberg-Riethmacher,E., Brinkmann,V., Yamaai,T., Lewin,G.R. and Birchmeier,C. (1997) Severe neuropathies in mice with targeted mutations of the erbB3 receptor. Nature, 389, 725–729. [DOI] [PubMed] [Google Scholar]
- Robinson M., Adu,J. and Davies,A.M. (1996a) Timing and regulation of trkB and BDNF mRNA expression in placode-derived sensory neurons and their targets. Eur. J. Neurosci., 8, 2399–2406. [DOI] [PubMed] [Google Scholar]
- Robinson M., Buj-Bello,A. and Davies,A.M. (1996b) Paracrine interactions of BDNF involving NGF-dependent embryonic sensory neurons. Mol. Cell. Neurosci., 7, 143–151. [DOI] [PubMed] [Google Scholar]
- Rohrer H., Heumann,R. and Thoenen,H. (1988) The synthesis of nerve growth factor (NGF) in developing skin is independent of innervation. Dev. Biol., 128, 240–244. [DOI] [PubMed] [Google Scholar]
- Roux P.P., Bhakar,A.L., Kennedy,T.E. and Barker,P.A. (2001) The p75 neurotrophin receptor activates Akt (protein kinase B) through a phosphatidylinositol 3-kinase-dependent pathway. J. Biol. Chem., 276, 23097–23104. [DOI] [PubMed] [Google Scholar]
- Ryden M., Hempstead,B. and Ibanez,C.F. (1997) Differential modulation of neuron survival during development by nerve growth factor binding to the p75 neurotrophin receptor. J. Biol. Chem., 272, 16322–16328. [DOI] [PubMed] [Google Scholar]
- Schwartz P.M., Borghesani,P.R., Levy,R.L., Pomeroy,S.L. and Segal,R.A. (1997) Abnormal cerebellar development and foliation in BDNF–/– mice reveals a role for neurotrophins in CNS patterning. Neuron, 19, 269–281. [DOI] [PubMed] [Google Scholar]
- Tafreshi A.P., Zhou,X.F. and Rush,R.A. (1998) Endogenous nerve growth factor and neurotrophin-3 act simultaneously to ensure the survival of postnatal sympathetic neurons in vivo. Neuroscience, 83, 373–380. [DOI] [PubMed] [Google Scholar]
- VanderZee C.E., Ross,G.M., Riopelle,R.J. and Hagg,T. (1996) Survival of cholinergic forebrain neurons in developing p75NGFR-deficient mice. Science, 274, 1729–1732. [DOI] [PubMed] [Google Scholar]
- Vogel K.S. and Davies,A.M. (1991) The duration of neurotrophic factor independence in early sensory neurons is matched to the time course of target field innervation. Neuron, 7, 819–830. [DOI] [PubMed] [Google Scholar]
- Vogel K.S. and Davies,A.M. (1993) Heterotopic transplantation of presumptive placodal ectoderm changes the fate of sensory neuron precursors. Development, 119, 263–276. [DOI] [PubMed] [Google Scholar]
- vonBartheld C.S. and Butowt,R. (2000) Expression of neurotrophin-3 (NT-3) and anterograde axonal transport of endogenous NT-3 by retinal ganglion cells in chick embryos. J. Neurosci., 20, 736–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H. and Tessier-Lavigne,M. (1999) En passant neurotrophic action of an intermediate axonal target in the developing mammalian CNS. Nature, 401, 765–769. [DOI] [PubMed] [Google Scholar]
- White F.A., Silos-Santiago,I., Molliver,D.C., Nishimura,M., Phillips,H., Barbacid,M. and Snider,W.D. (1996) Synchronous onset of NGF and TrkA survival dependence in developing dorsal root ganglia. J. Neurosci., 16, 4662–4672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson G.A., Fariñas,I., Backus,C., Yoshida,C.K. and Reichardt,L.F. (1996) Neurotrophin-3 is a survival factor in vivo for early mouse trigeminal neurons. J. Neurosci., 16, 7661–7669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright E.M., Vogel,K.S. and Davies,A.M. (1992) Neurotrophic factors promote the maturation of developing sensory neurons before they become dependent on these factors for survival. Neuron, 9, 139–150. [DOI] [PubMed] [Google Scholar]
- Wyatt S., Piñón,L.G.P., Ernfors,P. and Davies,A.M. (1997) Sympathetic neuron survival and TrkA expression in NT3-deficient mouse embryos. EMBO J., 16, 3115–3123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou X. and Rush,R.A. (1995) Sympathetic neurons in neonatal rats require endogenous neurotrophin-3 for survival. J. Neurosci., 15, 6521–6530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou X.F. and Rush,R.A. (1996) Endogenous brain-derived neurotrophic factor is anterogradely transported in primary sensory neurons. Neuroscience, 74, 945–953. [DOI] [PubMed] [Google Scholar]
- Zhou X.F., Chie,E.T., Deng,Y.S. and Rush,R.A. (1997) Rat mature sympathetic neurones derive neurotrophin 3 from peripheral effector tissues. Eur. J. Neurosci., 9, 2753–2764. [DOI] [PubMed] [Google Scholar]