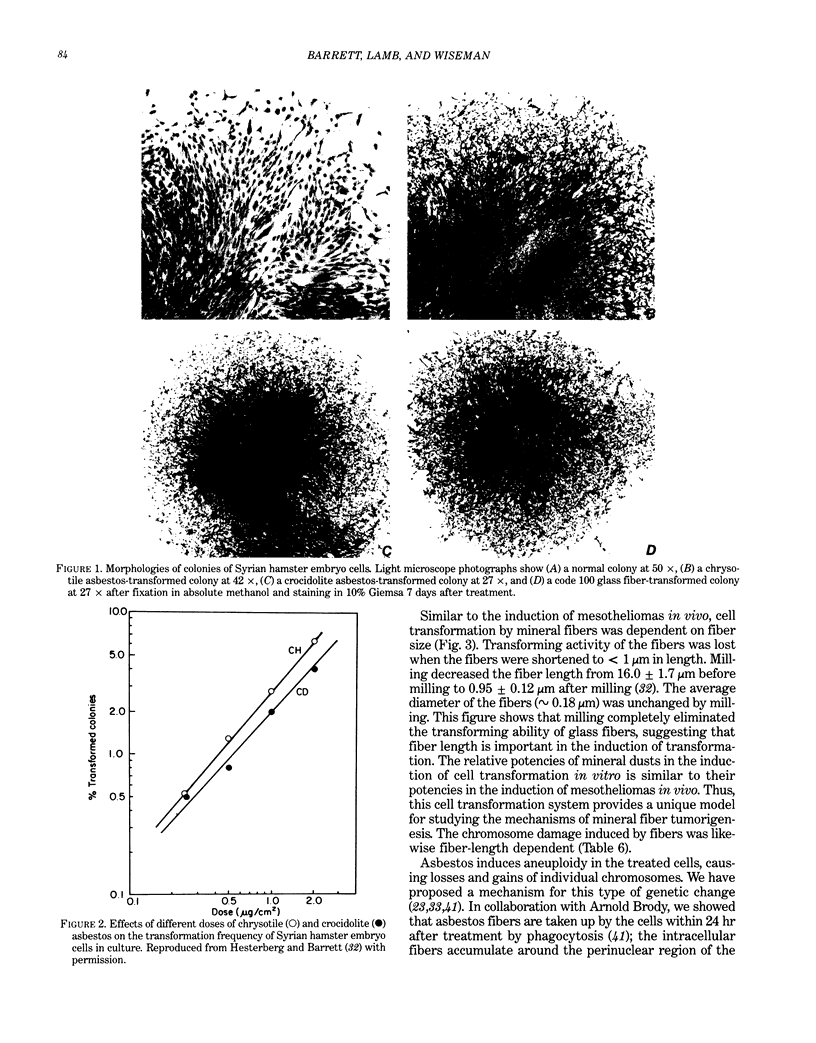
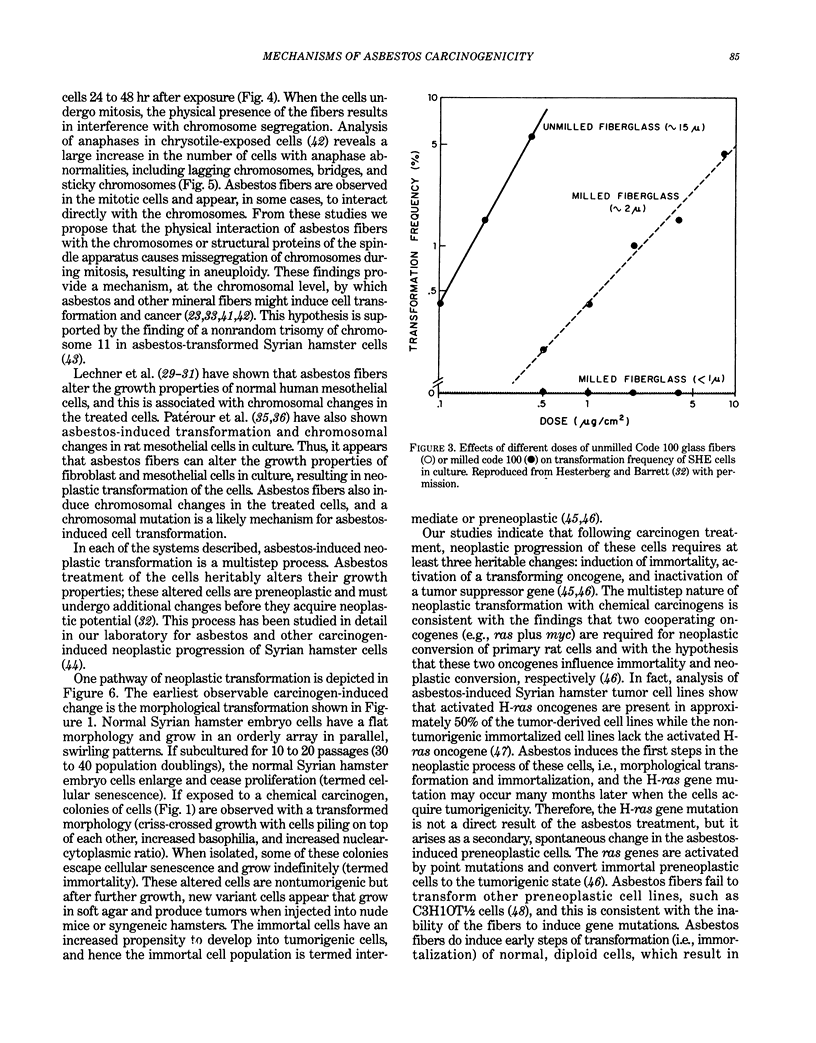
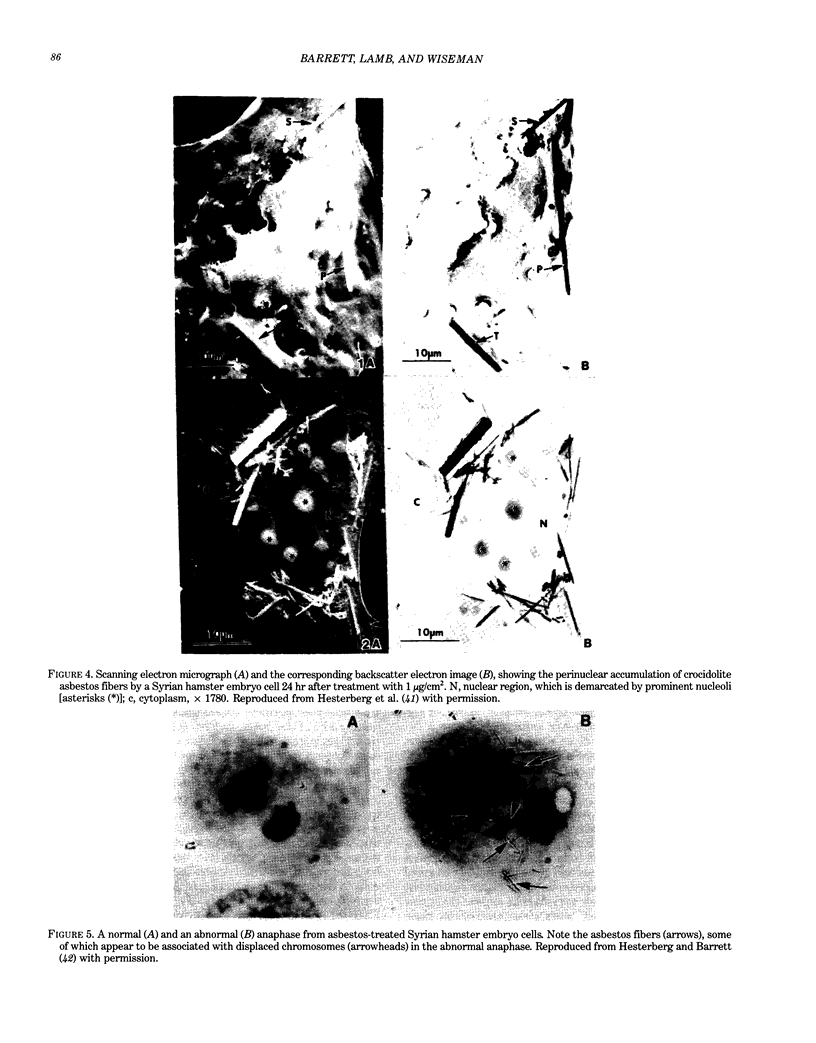
Abstract
Asbestos and other mineral fibers are carcinogenic to humans and animals but differ from many carcinogens in that they do not induce gene mutations. An understanding of these interesting human carcinogens, therefore, is an important problem in cancer research. Asbestos and other fibers induce predominantly two types of cancers: mesotheliomas and bronchogenic carcinomas. Fiber size is an important factor in the carcinogenic activity of these substances as has been shown for mesothelioma induction. For bronchogenic carcinomas, but not for mesotheliomas, a synergistic effect of asbestos exposure and cigarette smoke has been observed in humans. The mechanisms by which fibers alone versus fibers in concert with other carcinogens induce cancers are probably distinct. In addition to fiber dimensions, fiber durability and surface properties of fibers are important properties affecting carcinogenicity. Evidence exists that asbestos is a complete carcinogen, an initiator and a promoter. Multiple mechanisms must be operative to explain the diverse effects of mineral fibers. Although asbestos is inactive as a gene mutagen, there is now clear evidence that it induces chromosomal mutations (aneuploidy and aberrations) in a wide variety of mammalian cells including mesothelial cells. Asbestos also induces transformation of cells in culture including mesothelial cells and fibroblasts. A mechanism for cell transformation, which is dependent on fiber dimension, has been proposed. The fibers are phagocytized by the cells and accumulate in the perinuclear region of the cells. When the cell undergoes mitosis, the physical presence of the fibers interferes with chromosome segregation and results in anaphase abnormalities. The transformed cells show aneuploidy and other chromosome abnormalities.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Babu K. A., Lakkad B. C., Nigam S. K., Bhatt D. K., Karnik A. B., Thakore K. N., Kashyap S. K., Chatterjee S. K. In vitro cytological and cytogenetic effects of an Indian variety of chrysotile asbestos. Environ Res. 1980 Apr;21(2):416–422. doi: 10.1016/0013-9351(80)90045-6. [DOI] [PubMed] [Google Scholar]
- Barrett J. C., Oshimura M., Koi M. Role of oncogenes and tumor suppressor genes in a multistep model of carcinogenesis. Symp Fundam Cancer Res. 1986;39:45–56. [PubMed] [Google Scholar]
- Bortz W. M. Predictability of weight loss. JAMA. 1968 Apr 8;204(2):101–105. [PubMed] [Google Scholar]
- Brown R. C., Poole A., Fleming G. T. The influence of asbestos dust on the oncogenic transformation of C3H10T 1/2 cells. Cancer Lett. 1983 Mar;18(2):221–227. doi: 10.1016/0304-3835(83)90071-x. [DOI] [PubMed] [Google Scholar]
- Chamberlain M., Tarmy E. M. Asbestos and glass fibres in bacterial mutation tests. Mutat Res. 1977 May;43(2):159–164. doi: 10.1016/0027-5107(77)90001-x. [DOI] [PubMed] [Google Scholar]
- Gibas Z., Li F. P., Antman K. H., Bernal S., Stahel R., Sandberg A. A. Chromosome changes in malignant mesothelioma. Cancer Genet Cytogenet. 1986 Feb 15;20(3-4):191–201. doi: 10.1016/0165-4608(86)90074-9. [DOI] [PubMed] [Google Scholar]
- Hammond E. C., Selikoff I. J., Seidman H. Asbestos exposure, cigarette smoking and death rates. Ann N Y Acad Sci. 1979;330:473–490. doi: 10.1111/j.1749-6632.1979.tb18749.x. [DOI] [PubMed] [Google Scholar]
- Harington J. S., Allison A. C., Badami D. V. Mineral fibers: chemical, physicochemical, and biological properties. Adv Pharmacol Chemother. 1975;12(0):291–402. doi: 10.1016/s1054-3589(08)60223-9. [DOI] [PubMed] [Google Scholar]
- Harington J. S. Fiber carcinogenesis: epidemiologic observations and the Stanton hypothesis. J Natl Cancer Inst. 1981 Nov;67(5):977–989. [PubMed] [Google Scholar]
- Hesterberg T. W., Barrett J. C. Dependence of asbestos- and mineral dust-induced transformation of mammalian cells in culture on fiber dimension. Cancer Res. 1984 May;44(5):2170–2180. [PubMed] [Google Scholar]
- Hesterberg T. W., Barrett J. C. Induction by asbestos fibers of anaphase abnormalities: mechanism for aneuploidy induction and possibly carcinogenesis. Carcinogenesis. 1985 Mar;6(3):473–475. doi: 10.1093/carcin/6.3.473. [DOI] [PubMed] [Google Scholar]
- Hesterberg T. W., Butterick C. J., Oshimura M., Brody A. R., Barrett J. C. Role of phagocytosis in Syrian hamster cell transformation and cytogenetic effects induced by asbestos and short and long glass fibers. Cancer Res. 1986 Nov;46(11):5795–5802. [PubMed] [Google Scholar]
- Jaurand M. C., Kheuang L., Magne L., Bignon J. Chromosomal changes induced by chrysotile fibres or benzo-3,4-pyrene in rat pleural mesothelial cells. Mutat Res. 1986 Mar;169(3):141–148. doi: 10.1016/0165-1218(86)90093-5. [DOI] [PubMed] [Google Scholar]
- Klein G. The approaching era of the tumor suppressor genes. Science. 1987 Dec 11;238(4833):1539–1545. doi: 10.1126/science.3317834. [DOI] [PubMed] [Google Scholar]
- Koi M., Barrett J. C. Loss of tumor-suppressive function during chemically induced neoplastic progression of Syrian hamster embryo cells. Proc Natl Acad Sci U S A. 1986 Aug;83(16):5992–5996. doi: 10.1073/pnas.83.16.5992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kok K., Osinga J., Carritt B., Davis M. B., van der Hout A. H., van der Veen A. Y., Landsvater R. M., de Leij L. F., Berendsen H. H., Postmus P. E. Deletion of a DNA sequence at the chromosomal region 3p21 in all major types of lung cancer. Nature. 1987 Dec 10;330(6148):578–581. doi: 10.1038/330578a0. [DOI] [PubMed] [Google Scholar]
- Lavappa K. S., Fu M. M., Epstein S. S. Cytogenetic studies on chrysotile asbestos. Environ Res. 1975 Oct;10(2):165–173. doi: 10.1016/0013-9351(75)90080-8. [DOI] [PubMed] [Google Scholar]
- Lechner J. F., Tokiwa T., LaVeck M., Benedict W. F., Banks-Schlegel S., Yeager H., Jr, Banerjee A., Harris C. C. Asbestos-associated chromosomal changes in human mesothelial cells. Proc Natl Acad Sci U S A. 1985 Jun;82(11):3884–3888. doi: 10.1073/pnas.82.11.3884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan A., Davies P., Wagner J. C., Berry G., Holmes A. The biological effects of magnesium-leached chrysotile asbestos. Br J Exp Pathol. 1977 Oct;58(5):465–473. [PMC free article] [PubMed] [Google Scholar]
- Mossman B. T., Craighead J. E., MacPherson B. V. Asbestos-induced epithelial changes in organ cultures of hamster trachea: inhibition by retinyl methyl ether. Science. 1980 Jan 18;207(4428):311–313. doi: 10.1126/science.7350661. [DOI] [PubMed] [Google Scholar]
- Mossman B. T., Marsh J. P., Shatos M. A., Doherty J., Gilbert R., Hill S. Implication of active oxygen species as second messengers of asbestos toxicity. Drug Chem Toxicol. 1987;10(1-2):157–180. doi: 10.3109/01480548709042587. [DOI] [PubMed] [Google Scholar]
- Mossman B., Light W., Wei E. Asbestos: mechanisms of toxicity and carcinogenicity in the respiratory tract. Annu Rev Pharmacol Toxicol. 1983;23:595–615. doi: 10.1146/annurev.pa.23.040183.003115. [DOI] [PubMed] [Google Scholar]
- Oshimura M., Hesterberg T. W., Barrett J. C. An early, nonrandom karyotypic change in immortal Syrian hamster cell lines transformed by asbestos: trisomy of chromosome 11. Cancer Genet Cytogenet. 1986 Jul;22(3):225–237. doi: 10.1016/0165-4608(86)90159-7. [DOI] [PubMed] [Google Scholar]
- Paterour M. J., Bignon J., Jaurand M. C. In vitro transformation of rat pleural mesothelial cells by chrysotile fibres and/or benzo[a]pyrene. Carcinogenesis. 1985 Apr;6(4):523–529. doi: 10.1093/carcin/6.4.523. [DOI] [PubMed] [Google Scholar]
- Peto J., Seidman H., Selikoff I. J. Mesothelioma mortality in asbestos workers: implications for models of carcinogenesis and risk assessment. Br J Cancer. 1982 Jan;45(1):124–135. doi: 10.1038/bjc.1982.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peto J., Seidman H., Selikoff I. J. Mesothelioma mortality in asbestos workers: implications for models of carcinogenesis and risk assessment. Br J Cancer. 1982 Jan;45(1):124–135. doi: 10.1038/bjc.1982.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popescu N. C., Chahinian A. P., DiPaolo J. A. Nonrandom chromosome alterations in human malignant mesothelioma. Cancer Res. 1988 Jan 1;48(1):142–147. [PubMed] [Google Scholar]
- Sincock A., Seabright M. Induction of chromosome changes in Chinese hamster cells by exposure to asbestos fibres. Nature. 1975 Sep 4;257(5521):56–58. doi: 10.1038/257056a0. [DOI] [PubMed] [Google Scholar]
- Spurny K. R. Natural fibrous zeolites and their carcinogenicity-a review. Sci Total Environ. 1983 Sep;30:147–166. doi: 10.1016/0048-9697(83)90008-6. [DOI] [PubMed] [Google Scholar]
- Stanton M. F., Layard M., Tegeris A., Miller E., May M., Morgan E., Smith A. Relation of particle dimension to carcinogenicity in amphibole asbestoses and other fibrous minerals. J Natl Cancer Inst. 1981 Nov;67(5):965–975. [PubMed] [Google Scholar]
- Suzuki Y., Kohyama N. Malignant mesothelioma induced by asbestos and zeolite in the mouse peritoneal cavity. Environ Res. 1984 Oct;35(1):277–292. doi: 10.1016/0013-9351(84)90136-1. [DOI] [PubMed] [Google Scholar]
- Valerio F., De Ferrari M., Ottaggio L., Repetto E., Santi L. Cytogenetic effects of Rhodesian chrysotile on human lymphocytes in vitro. IARC Sci Publ. 1980;(30):485–489. [PubMed] [Google Scholar]
- Wagner J. C., Berry G., Skidmore J. W., Timbrell V. The effects of the inhalation of asbestos in rats. Br J Cancer. 1974 Mar;29(3):252–269. doi: 10.1038/bjc.1974.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner J. C., Skidmore J. W., Hill R. J., Griffiths D. M. Erionite exposure and mesotheliomas in rats. Br J Cancer. 1985 May;51(5):727–730. doi: 10.1038/bjc.1985.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whang-Peng J., Kao-Shan C. S., Lee E. C., Bunn P. A., Carney D. N., Gazdar A. F., Minna J. D. Specific chromosome defect associated with human small-cell lung cancer; deletion 3p(14-23). Science. 1982 Jan 8;215(4529):181–182. doi: 10.1126/science.6274023. [DOI] [PubMed] [Google Scholar]