Abstract
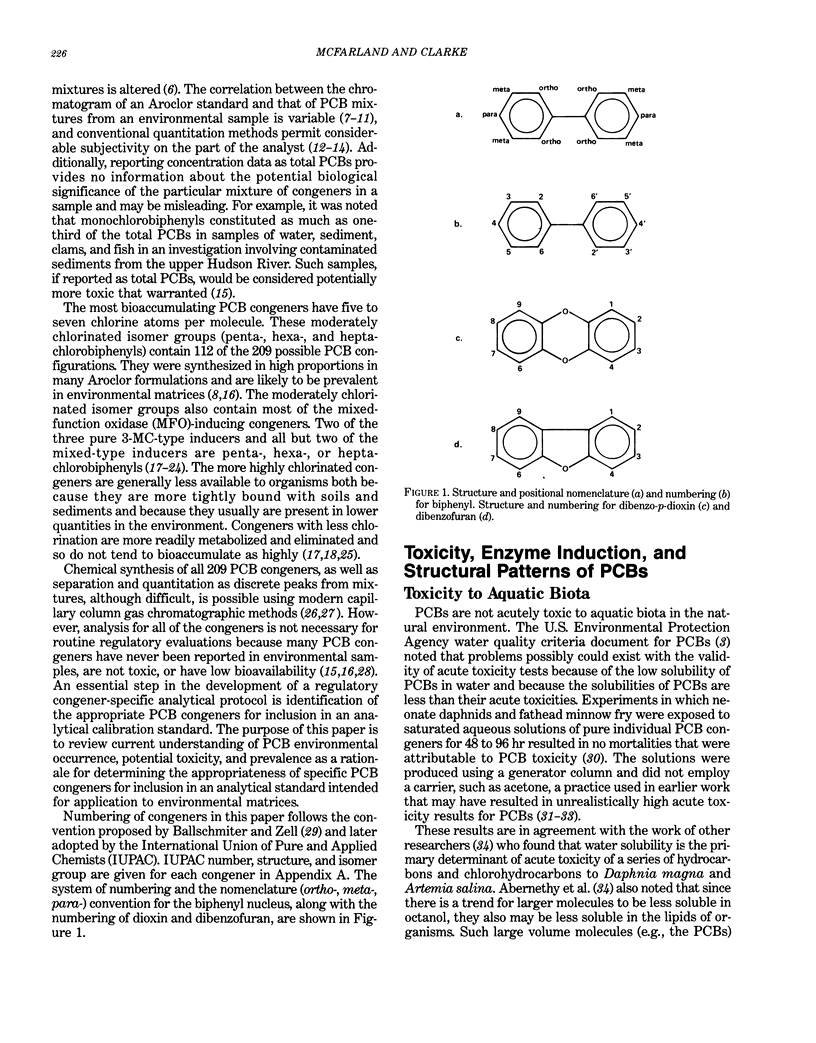
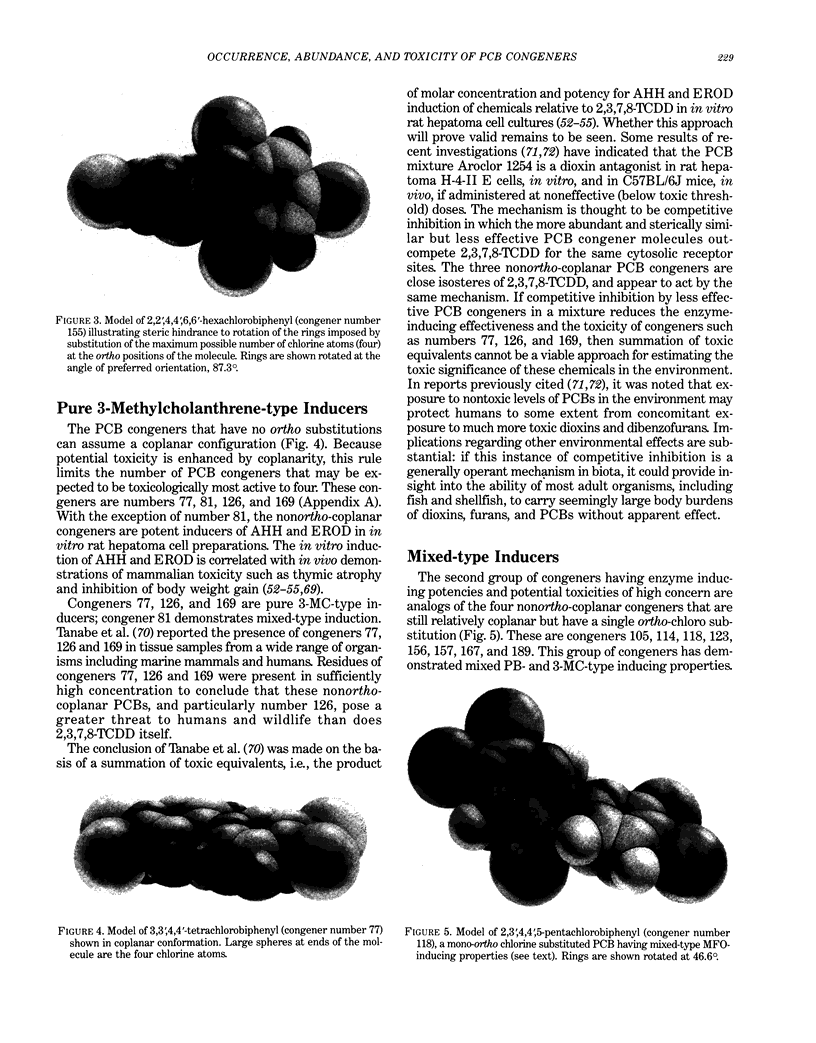
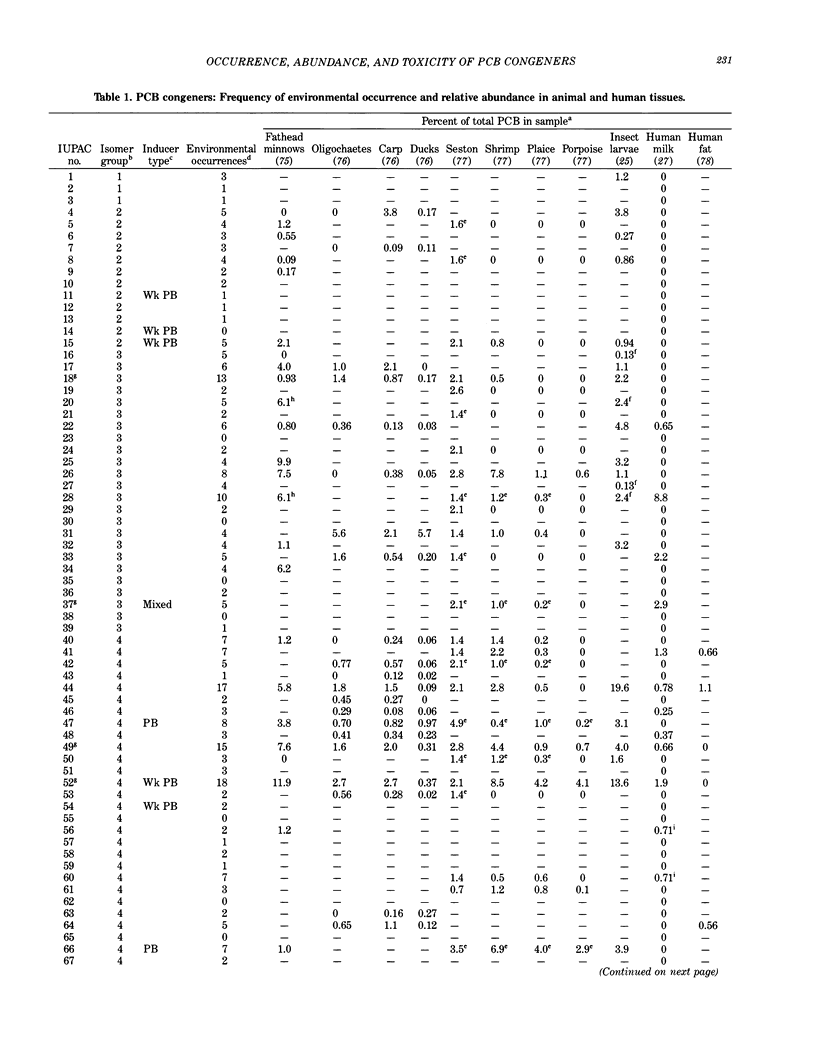
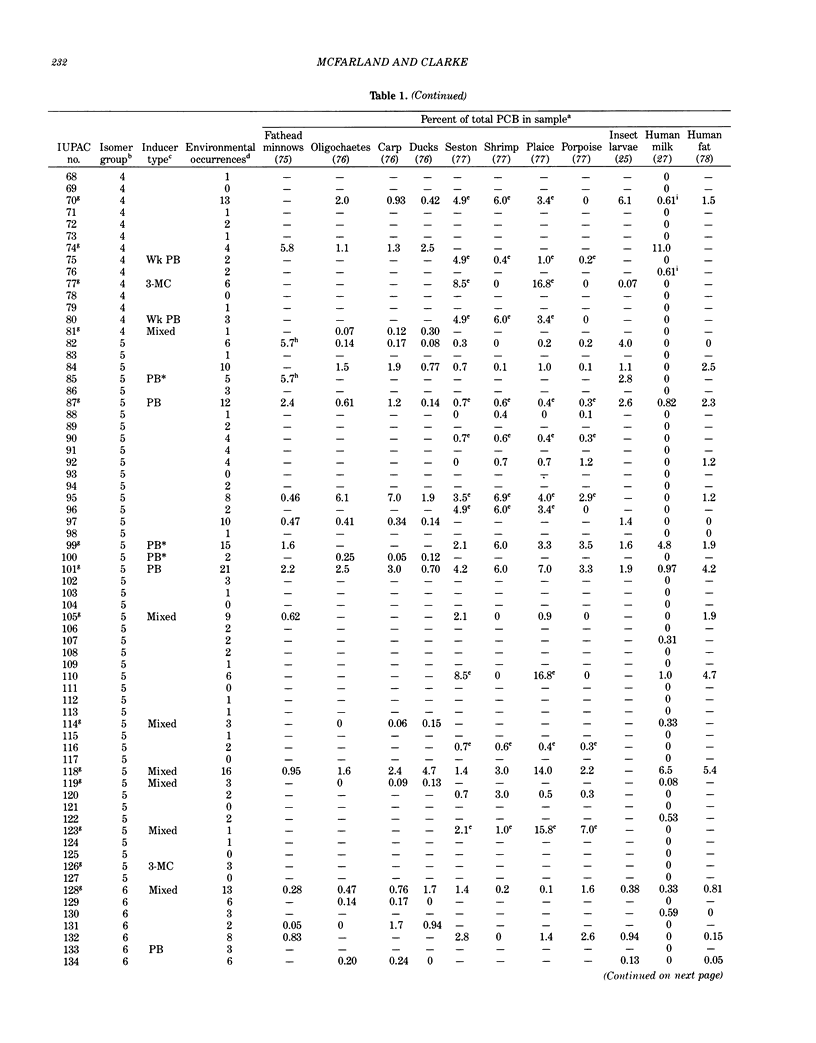
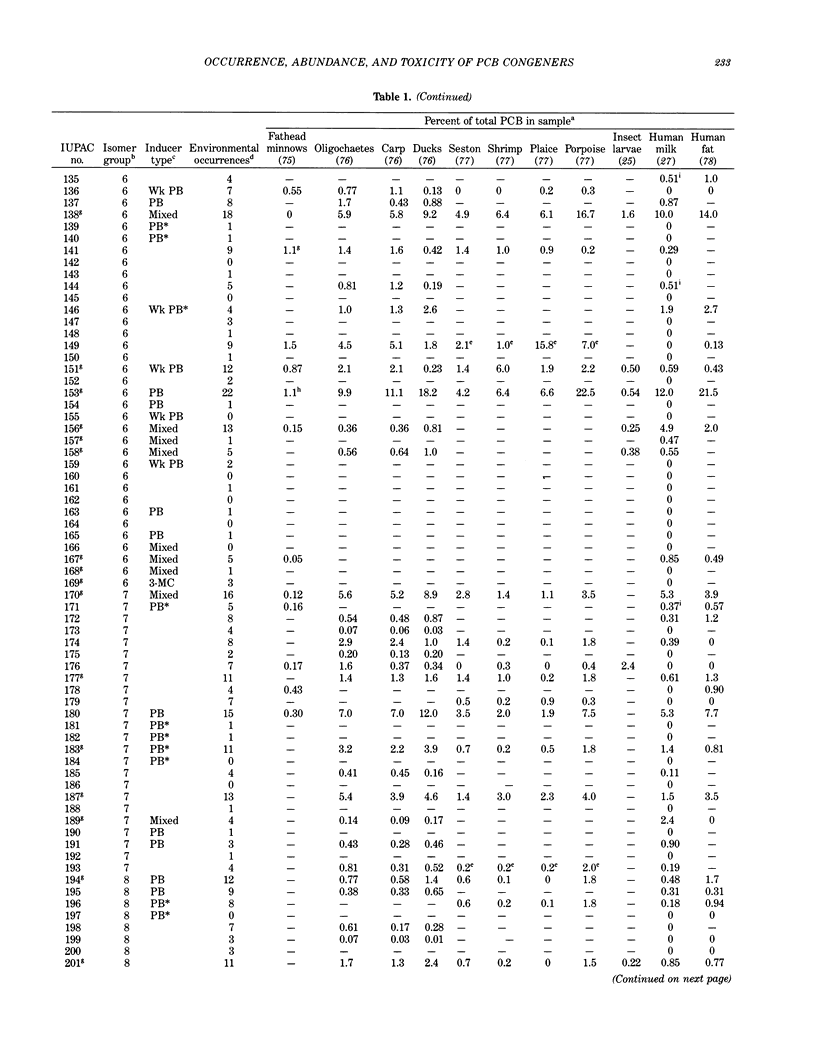
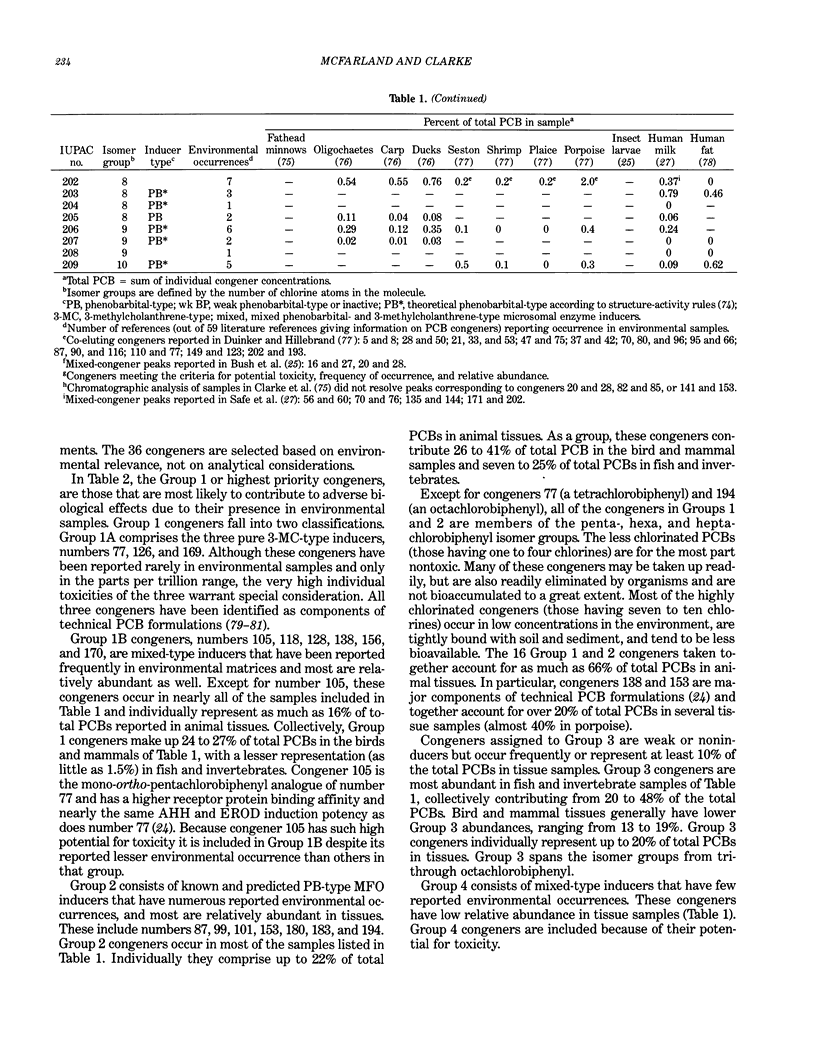
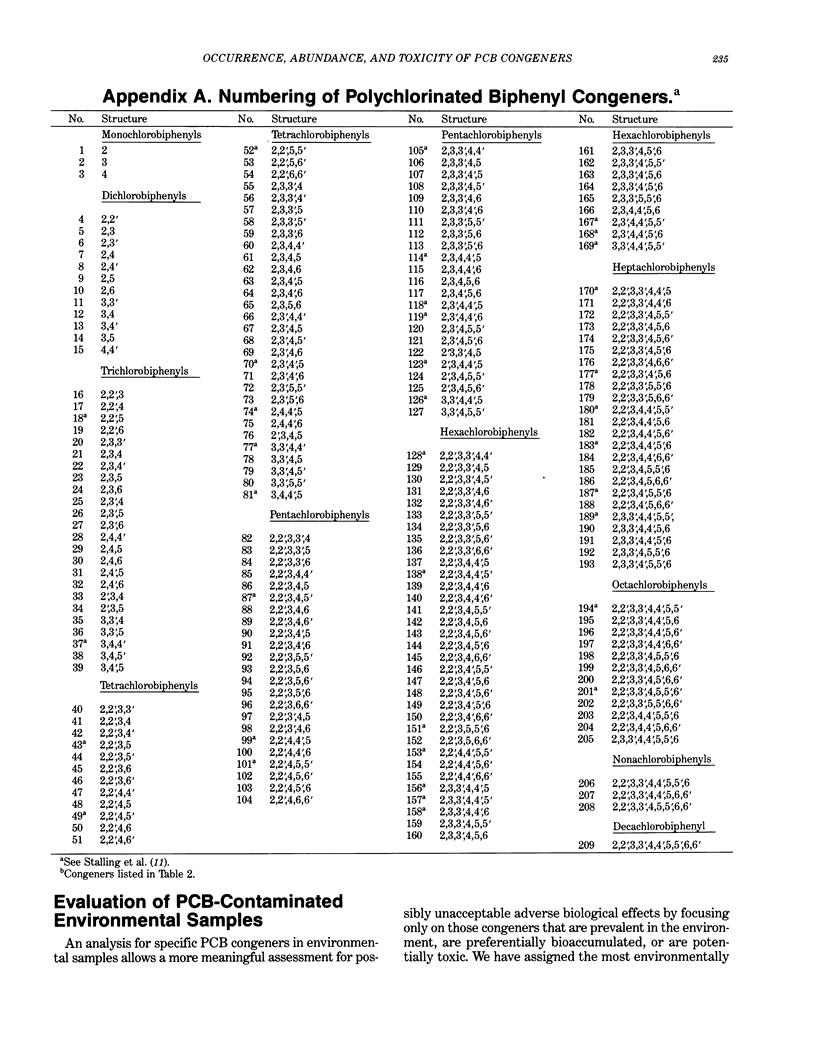
Polychlorinated biphenyls (PCBs) as environmental contaminants often cannot be adequately described by reference to Aroclors or to total PCBs. Although there are 209 possible PCB configurations (congeners), perhaps half that number account for nearly all of the environmental contamination attributable to PCBs. Still fewer congeners are both prevalent and either demonstrably or potentially toxic. If potential toxicity, environmental prevalence, and relative abundance in animal tissues are used as criteria, the number of environmentally threatening PCB congeners reduces to about thirty-six. Twenty-five of these account for 50 to 75% of total PCBs in tissue samples of fish, invertebrates, birds, and mammals. A few PCB congeners that are sterically similar to 2,3,7,8-tetrachlorodibenzo-p-dioxin (2,3,7,8-TCDD) are directly toxic. Other PCB congeners, as well as those that are directly toxic, may also be involved in toxicity indirectly by stimulating the production of (inducing) bioactivating enzyme systems. The most consequential of these have the ability to induce aryl hydrocarbon metabolizing mixed-function oxidases (MFOs). A result can be an increased capacity for bioactivation of otherwise nontoxic foreign compounds such as certain polynuclear aromatic hydrocarbons (PAH) to cytotoxic or genotoxic metabolites. The effectiveness of specific PCB congeners as inducers of different types of cytochrome P-450-dependent MFO systems is determined by their stereochemistry. Although MFO induction is not a proximate cause, it is a strong correlate of certain kinds of toxicities. Structural patterns can thus be used to discriminate among PCB congeners on the basis of toxic potential, if not entirely on toxicity per se. Congeners that demonstrate 3-methylcholanthrene-type (3-MC-type) and mixed-type MFO induction have the greatest toxic potential. These congeners most closely resemble 2,3,7,8-TCDD in their structures and in their toxic effects. The larger group of phenobarbital-type (PB-type) inducers have considerably less potential for contributing to toxic effects. Weak inducers and noninducing congeners have the least potential for toxicity. Using the rationale described in this paper, we assigned the most evironmentally threatening PCB congeners to four groups. Congeners assigned to Group 1 are considered most likely to contribute to adverse biological effects attributable to PCBs in an environmental sample. Group 1A contains the three most potent (pure 3-MC-type inducer) congeners, IUPAC numbers 77, 126, and 169. Six congeners, numbers 105, 118, 128, 138, 156, and 170, are assigned to Group 1B. These congeners are mixed-type inducers that have been reported frequently in environmental samples.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albro P. W., McKinney J. D. The relationship between polarizability of polychlorinated biphenyls and their induction of mixed function oxidase activity. Chem Biol Interact. 1981 Mar 15;34(3):373–378. doi: 10.1016/0009-2797(81)90109-5. [DOI] [PubMed] [Google Scholar]
- Bannister R., Davis D., Zacharewski T., Tizard I., Safe S. Aroclor 1254 as a 2,3,7,8-tetrachlorodibenzo-p-dioxin antagonist: effects on enzyme induction and immunotoxicity. Toxicology. 1987 Oct 12;46(1):29–42. doi: 10.1016/0300-483x(87)90135-1. [DOI] [PubMed] [Google Scholar]
- Bunyan P. J., Page J. M. Polychlorinated biphenyls. The effect of structure on the induction of quail hepatic microsomal enzymes. Toxicol Appl Pharmacol. 1978 Mar;43(3):507–518. doi: 10.1016/s0041-008x(78)80010-6. [DOI] [PubMed] [Google Scholar]
- Bush B., Simpson K. W., Shane L., Koblintz R. R. PCB congener analysis of water and caddisfly larvae (Insecta:Trichoptera) in the upper Hudson River by glass capillary chromatography. Bull Environ Contam Toxicol. 1985 Jan;34(1):96–105. doi: 10.1007/BF01609708. [DOI] [PubMed] [Google Scholar]
- Casterline J. L., Jr, Bradlaw J. A., Puma B. J., Ku Y. Screening of fresh water fish extracts for enzyme-inducing substances by an aryl hydrocarbon hydroxylase induction bioassay technique. J Assoc Off Anal Chem. 1983 Sep;66(5):1136–1139. [PubMed] [Google Scholar]
- Duinker J. C., Hillebrand M. T. Composition of PCB mixtures in biotic and abiotic marine compartments (Dutch Wadden Sea). Bull Environ Contam Toxicol. 1983 Jul;31(1):25–32. doi: 10.1007/BF01608762. [DOI] [PubMed] [Google Scholar]
- Fingerman S. W., Fingerman M. Effects of a polychlorinated biphenyl and a polychlorinated dibenzofuran on molting of the fiddler crab, Uca pugilator. Bull Environ Contam Toxicol. 1977 Aug;18(2):138–142. doi: 10.1007/BF01686320. [DOI] [PubMed] [Google Scholar]
- Goldstein J. A., Hickman P., Bergman H., McKinney J. D., Walker M. P. Separation of pure polychlorinated biphenyl isomers into two types of inducers on the basis of induction of cytochrome P-450 or P-448. Chem Biol Interact. 1977 Apr;17(1):69–87. doi: 10.1016/0009-2797(77)90073-4. [DOI] [PubMed] [Google Scholar]
- Goldstein J. A. The structure-activity relationships of halogenated biphenyls as enzyme inducers. Ann N Y Acad Sci. 1979 May 31;320:164–178. [PubMed] [Google Scholar]
- Haake J. M., Safe S., Mayura K., Phillips T. D. Aroclor 1254 as an antagonist of the teratogenicity of 2,3,7,8-tetrachlorodibenzo-p-dioxin. Toxicol Lett. 1987 Oct;38(3):299–306. doi: 10.1016/0378-4274(87)90012-9. [DOI] [PubMed] [Google Scholar]
- James M. O. Conjugation of organic pollutants in aquatic species. Environ Health Perspect. 1987 Apr;71:97–103. doi: 10.1289/ehp.877197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones K. C. Determination of polychlorinated biphenyls in human foodstuffs and tissues: suggestions for a selective congener analytical approach. Sci Total Environ. 1988 Jan;68:141–159. doi: 10.1016/0048-9697(88)90367-1. [DOI] [PubMed] [Google Scholar]
- Kaminsky L. S., Kennedy M. W., Adams S. M., Guengerich F. P. Metabolism of dichlorobiphenyls by highly purified isozymes of rat liver cytochrome P-450. Biochemistry. 1981 Dec 22;20(26):7379–7384. doi: 10.1021/bi00529a009. [DOI] [PubMed] [Google Scholar]
- Kamops L. R., Trotter W. J., Young S. J., Smith A. C., Roach J. A., Page S. W. Separation and quantitation of 3,3',4,4'-tetrachlorobiphenyl and 3,3',4,4',5,5'-hexachlorobiphenyl in aroclors using florisil column chromatography and gas-liquid chromatography. Bull Environ Contam Toxicol. 1979 Sep;23(1-2):51–56. doi: 10.1007/BF01769915. [DOI] [PubMed] [Google Scholar]
- Kimbrough R. D. The toxicity of polychlorinated polycyclic compounds and related chemicals. CRC Crit Rev Toxicol. 1974 Jan;2(4):445–498. doi: 10.3109/10408447309025705. [DOI] [PubMed] [Google Scholar]
- Kleinow K. M., Melancon M. J., Lech J. J. Biotransformation and induction: implications for toxicity, bioaccumulation and monitoring of environmental xenobiotics in fish. Environ Health Perspect. 1987 Apr;71:105–119. doi: 10.1289/ehp.8771105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau S. S., Abrams G. D., Zannoni V. G. Metabolic activation and detoxification of bromobenzene leading to cytotoxicity. J Pharmacol Exp Ther. 1980 Sep;214(3):703–708. [PubMed] [Google Scholar]
- Lau S. S., Zannoni V. G. Bromobenzene metabolism in the rabbit: specific forms of cytochrome P-450 involved in 2,3- and 3,4-epoxidation. Mol Pharmacol. 1981 Jul;20(1):234–235. [PubMed] [Google Scholar]
- Lau S. S., Zannoni V. G. Hepatic microsomal epoxidation of bromobenzene to phenols and its toxicological implication. Toxicol Appl Pharmacol. 1979 Sep 15;50(2):309–318. doi: 10.1016/0041-008x(79)90156-x. [DOI] [PubMed] [Google Scholar]
- Matthews H., Fries G., Gardner A., Garthoff L., Goldstein J., Ku Y., Moore J. Metabolism and biochemical toxicity of PCBs and PBBs. Environ Health Perspect. 1978 Jun;24:147–155. doi: 10.1289/ehp.7824147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews H., Fries G., Gardner A., Garthoff L., Goldstein J., Ku Y., Moore J. Metabolism and biochemical toxicity of PCBs and PBBs. Environ Health Perspect. 1978 Jun;24:147–155. doi: 10.1289/ehp.7824147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKinney J. D., Singh P. Structure-activity relationships in halogenated biphenyls: unifying hypothesis for structural specificity. Chem Biol Interact. 1981 Jan;33(2-3):271–283. doi: 10.1016/0009-2797(81)90046-6. [DOI] [PubMed] [Google Scholar]
- Nebert D. W., Jensen N. M. The Ah locus: genetic regulation of the metabolism of carcinogens, drugs, and other environmental chemicals by cytochrome P-450-mediated monooxygenases. CRC Crit Rev Biochem. 1979;6(4):401–437. doi: 10.3109/10409237909105427. [DOI] [PubMed] [Google Scholar]
- Parkinson A., Cockerline R., Safe S. Polychlorinated biphenyl isomers and congeners as inducers of both 3-methylcholanthrene- and phenobarbitone-type microsomal enzyme activity. Chem Biol Interact. 1980 Mar;29(3):277–289. doi: 10.1016/0009-2797(80)90147-7. [DOI] [PubMed] [Google Scholar]
- Parkinson A., Robertson L. W., Safe L., Safe S. Polychlorinated biphenyls as inducers of hepatic microsomal enzymes: effects of di-ortho substitution. Chem Biol Interact. 1981 Apr;35(1):1–12. doi: 10.1016/0009-2797(81)90059-4. [DOI] [PubMed] [Google Scholar]
- Parkinson A., Robertson L., Safe L., Safe S. Polychlorinated biphenyls as inducers of hepatic microsomal enzymes: structure-activity rules. Chem Biol Interact. 1980 Jun;30(3):271–285. doi: 10.1016/0009-2797(80)90050-2. [DOI] [PubMed] [Google Scholar]
- Parkinson A., Safe S. H., Robertson L. W., Thomas P. E., Ryan D. E., Reik L. M., Levin W. Immunochemical quantitation of cytochrome P-450 isozymes and epoxide hydrolase in liver microsomes from polychlorinated or polybrominated biphenyl-treated rats. A study of structure-activity relationships. J Biol Chem. 1983 May 10;258(9):5967–5976. [PubMed] [Google Scholar]
- Safe S., Bandiera S., Sawyer T., Robertson L., Safe L., Parkinson A., Thomas P. E., Ryan D. E., Reik L. M., Levin W. PCBs: structure-function relationships and mechanism of action. Environ Health Perspect. 1985 May;60:47–56. doi: 10.1289/ehp.856047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawyer T., Safe S. PCB isomers and congeners: induction of aryl hydrocarbon hydroxylase and ethoxyresorufin O-deethylase enzyme activities in rat hepatoma cells. Toxicol Lett. 1982 Sep;13(1-2):87–93. doi: 10.1016/0378-4274(82)90142-4. [DOI] [PubMed] [Google Scholar]
- Stalling D. L., Schwartz T. R., Dunn W. J., 3rd, Wold S. Classification of polychlorinated biphenyl residues: isomers vs. homologue concentrations in modeling aroclors and polychlorinated biphenyl residues. Anal Chem. 1987 Jul 15;59(14):1853–1859. doi: 10.1021/ac00141a026. [DOI] [PubMed] [Google Scholar]
- Stegeman J. J., Kloepper-Sams P. J. Cytochrome P-450 isozymes and monooxygenase activity in aquatic animals. Environ Health Perspect. 1987 Apr;71:87–95. doi: 10.1289/ehp.877187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanabe S., Kannan N., Subramanian A., Watanabe S., Tatsukawa R. Highly toxic coplanar PCBs: occurrence, source, persistency and toxic implications to wildlife and humans. Environ Pollut. 1987;47(2):147–163. doi: 10.1016/0269-7491(87)90044-3. [DOI] [PubMed] [Google Scholar]
- Webb R. G., McCall A. C. Quantitative PCB standards for electron capture gas chromatography. J Chromatogr Sci. 1973 Jul;11(7):366–373. doi: 10.1093/chromsci/11.7.366. [DOI] [PubMed] [Google Scholar]







