Abstract
Survivin is an anti-apoptotic protein that is overexpressed in most human cancers. We show that survivin forms complexes with a cellular protein, hepatitis B X-interacting protein (HBXIP), which was originally recognized for its association with the X protein of hepatitis B virus (HBX). Survivin–HBXIP complexes, but neither survivin nor HBXIP individually, bind pro-caspase-9, preventing its recruitment to Apaf1, and thereby selectively suppressing apoptosis initiated via the mitochondria/cytochrome c pathway. Viral HBX protein also interacts with the survivin– HBXIP complex and suppresses caspase activation in a survivin-dependent manner. Thus, HBXIP functions as a cofactor for survivin, and serves as a link between the cellular apoptosis machinery and a viral pathogen involved in hepatocellular carcinogenesis.
Keywords: apoptosis/caspase/hepatitis B virus/hepatitis B X-interacting protein/survivin
Introduction
SURVIVIN represents one of the most tumor-specific genes in the human genome (Velculescu et al., 1999). The 17 kDa survivin protein is scarcely expressed in normal adult tissues, but is found at high levels in most human cancers (Ambrosini et al., 1997). Normally, survivin is expressed only during late stages of the cell cycle (particularly mitosis and anaphase), where it associates with the mitotic spindle and related structures, performing functions important for chromosome segregation and cytokinesis (Li et al., 1998; Reed and Bischoff, 2000). However, many cancers contain constitutively high levels of cytosolic p17 survivin, and overexpression of this protein blocks apoptosis both in vitro in cultured cells and in vivo in transgenic mice through poorly defined mechanisms (Ambrosini et al., 1997; Grossman et al., 2001; Reed, 2001).
Survivin contains a characteristic zinc-binding fold called the BIR domain. Many BIR-containing proteins suppress apoptosis when overexpressed, thus prompting the term inhibitor of apoptosis proteins (IAPs). The principal mechanism of apoptosis suppression by IAP family members, such as XIAP, has been defined. These proteins directly bind and suppress the activity of caspases (Deveraux and Reed, 1999), intracellular proteases responsible for apoptosis (reviewed in Cryns and Yuan, 1998). Although some studies have suggested that p17 survivin also binds and suppresses caspases, others have failed to demonstrate direct effects on these proteases (Banks et al., 2000; Verdecia et al., 2000; Shin et al., 2001).
To provide an insight into the anti-apoptotic mechanism of survivin, we screened cDNA libraries for survivin-binding proteins, resulting in the discovery that hepatitis B X-interacting protein (HBXIP) associates with survivin. HBXIP was originally identified by its interaction with the HBX protein of hepatitis B virus (HBV) (Melegari et al., 1998), a putative oncogenic protein previously implicated in apoptosis regulation and other processes (reviewed in Murakami, 2001). We demonstrate here that HBXIP operates as a cofactor for survivin, allowing it to bind and suppress activation of pro-caspase-9, the apical protease in a mitochondrial pathway for cell death. The findings thus provide novel insights into the anti-apoptotic mechanism of survivin, and suggest a link between survivin and cancers associated with HBV infection.
Results
Survivin differs from XIAP in caspase inhibitory activity
We compared the effects of purified recombinant survivin and XIAP on recombinant active caspase-3 in vitro. While XIAP potently suppressed caspase-3, survivin displayed no ability to suppress caspase-3 protease activity, as measured by the hydrolysis of the fluorigenic peptide substrate acetyl-Asp-Glu-Val-Asp-aminofluorocoumarin (Ac-DEVD-AFC) (Figure 1A). Even at a 1000-fold molar excess of survivin relative to active caspase-3, essentially no inhibition was observed. Similar results were obtained using caspase-7 and -9, with either wild-type survivin or a T34E survivin mutant designed to mimic the phosphorylated form of survivin (O’Connor et al., 2000). Again, XIAP inhibited, but survivin did not (data not shown).
Fig. 1. Differences in caspase suppression by survivin and XIAP. Comparisons were made of the effects of purified survivin (left panels) and XIAP (middle panels) on (A) recombinant active caspase-3 or (B and C) caspase-3-like protease activity induced in cell extracts by Cyt-c. Caspase activity was continuously measured, based on cleavage of Ac-DEVD-AFC fluorigenic substrate [relative fluorescence units (RFU)/min/µg]. In (A), 100 pM recombinant active caspase-3 was incubated with recombinant survivin (left panel) or XIAP (middle panel) or with control protein. In (B), purified survivin (left panel) or XIAP (middle panel), or control protein was added to 293 cell lysates concurrently with Cyt-c and dATP. In (C), survivin (left panel) or XIAP (middle panel) was added to cell extracts after Cyt-c and dATP. Data shown in the panels on the right are normalized relative to control protein (mean ± SD; n = 3).
We then measured caspase activity in cell extracts, using exogenously supplied cytochrome c (Cyt-c) to trigger activation of caspases—a treatment known to cause Cyt-c-dependent oligomerization of the caspase activator Apaf1, which then binds and activates pro-caspase-9, followed by cleavage and activation of downstream protease caspase-3 (Zou et al., 1999). When purified recombinant survivin or XIAP was added to extracts prior to stimulation with Cyt-c, caspase activity in the cell lysates was suppressed in a concentration-dependent manner by both proteins (Figure 1B). However, if survivin or XIAP was added to extracts after stimulation with Cyt-c, then XIAP suppressed caspase activity, whereas survivin did not (Figure 1C). Although higher amounts of survivin protein were required for caspase suppression, compared with XIAP, we noted that survivin is rapidly degraded in cytosolic extracts (see Supplementary figure 1 available at The EMBO Journal Online), making quantitative comparisons difficult.
Survivin binds HBXIP
Since survivin inhibits caspase activation in cell extracts but not when purified, we reasoned that it might require a cofactor. To identify potential partners of survivin, we performed yeast two-hybrid cDNA library screens using survivin as a bait. From a pool of 64 candidate clones, 17 represented cDNAs encoding the HBXIP protein, with all clones encoding full-length protein (see Supplementary figure 2). HBXIP is a 91-amino-acid protein widely expressed in human tissues whose function is unknown (Melegari et al., 1998). We confirmed the direct association of survivin and HBXIP by protein-binding assays (Figure 2A) using purified recombinant His6-HBXIP and untagged survivin.
Fig. 2. HBXIP directly binds survivin. (A) In vitro binding experiments were performed using either purified recombinant survivin (untagged) or GST–XIAP incubated with His6-HBXIP, His6-TRAF3 or SMAC-His6 immobilized on nickel beads. Bound proteins were analyzed by immunoblotting using anti-survivin (upper panel) or anti-XIAP (middle panel) antisera. His6-tagged proteins were also analyzed by SDS–PAGE/immunoblotting using an anti-His6 antibody (lower panel). (B) Various fragments of survivin were produced by in vitro translation with [35S]l-methionine. Survivin fragments were then incubated with GST–CD40 control protein or GST–HBXIP immobilized on glutathione–Sepharose. Bound proteins were analyzed by autoradiography. The BIR domain is encompassed in residues 15–89. (C and D) 293T cells were transiently transfected with plasmids encoding myc-tagged survivin, mutant survivin lacking the BIR domain (del-SVV) or myc–XIAP, together with FLAG–HBXIP or FLAG–SIP (used as a negative control). Lysates were subjected to immunoprecipitation using anti-FLAG antibody (C and D, upper panel) or anti-myc antibody (D, middle panel). Immunoprecipitates were analyzed by immunoblotting using anti-myc antibody (C and D, upper panel) or anti-FLAG antibody (D, middle panel). Lysates were also blotted by anti-myc (C, middle panel, and D, lower panel) or anti-FLAG antibodies (C, lower panel). (E) Lysates from untransfected HepG2 cells used for immunoprecipitation with rabbit anti-survivin antisera, followed by blotting with chicken anti-HBXIP or rabbit anti-survivin antibodies. (F) Lysates from unfractionated 293 cells (T) and from subcellular fractions (M, membrane; C, cytoplasmic; N, nuclear), normalized for cell equivalents, were analyzed by immunoblotting using anti-HBXIP and anti-survivin. Blotting using anti-Hsp60, anti-caspase-3 and anti-PARP antibodies was also performed as markers for membrane (mitochondria), cytosolic and nuclear proteins, respectively.
To map the region of survivin required for interaction with HBXIP, we prepared fragments of survivin by in vitro translation, and assayed their binding to purified GST–HBXIP. In this regard, survivin contains a single BIR (residues 15–88), followed by a dimerization domain (residues 89–126) and an α-helical region (residues 126–142) that mediates association with mitotic structures in dividing cells (Verdecia et al., 2000). Fragments of survivin retaining the BIR domain bound HBXIP in vitro, while fragments lacking it did not (Figure 2B). Thus, the BIR domain of survivin is necessary and sufficient for HBXIP binding in vitro.
HBXIP binding to survivin in cells was confirmed by co-immunoprecipitation assays (Figure 2C and D). HBXIP bound to survivin but not to XIAP, BIR-deficient survivin or various irrelevant proteins such as SIP (Siah-interacting protein), confirming the specificity of the interaction of HBXIP with survivin. In contrast, Leu48, Leu55 and Ile62 mutants affecting the putative leucine zipper of HBXIP retained their ability to bind survivin, suggesting that this motif is not essential (data not shown). Binding of endogenous HBXIP to endogenous survivin was also detected by co-immunoprecipitation assays, using anti-survivin antibody to immunoprecipitate survivin, followed by immunoblot analysis of the resulting immune complexes using chicken anti-HBXIP antiserum (Figure 2E). Moreover, subcellular fractionation experiments confirmed that survivin and HBXIP reside in the same cellular compartment, with both proteins found predominantly in the cytosolic fraction of cells (Figure 2F). By immunostaining of tumor tissues, we also observed localization of both survivin and HBXIP predominantly in the cytosol of interphase cells (data not shown).
HBXIP interaction with survivin is modulated by phosphorylation
Phosphorylation of survivin at Thr34, probably by Cdc2, is required for its anti-apoptotic activity (O’Connor et al., 2000). We therefore compared the ability of HBXIP to co-immunoprecipitate with wild-type (W) versus T34A mutant survivin (Figure 3A). Whereas HBXIP co-immunoprecipitated with survivin, it did not associate with survivin (T34A). To further explore the role of phosphorylation, we performed experiments where cells were transfected with dominant-negative Cdc2 (Figure 3B). Based on immunoblot analysis using a phospho-specific antibody that detects T34 phosphorylated survivin (O’Connor et al., 2000), expression of Cdc2 dominant-negative greatly reduced amounts of phosphorylated survivin in cells. In parallel, Cdc2 dominant-negative substantially reduced association of HBXIP with survivin, as demonstrated by co-immunoprecipitation assays. Moreover, a T34E phospho-mimic of survivin bound HBXIP with higher affinity compared with wild-type survivin in vitro (Figure 3C), confirming the importance of this residue in survivin for modulating interactions with HBXIP. We conclude, therefore, that phosphorylation of survivin is required for optimal association with HBXIP, presumably explaining the low proportion of total cellular HBXIP and survivin interacting in various binding assays (Figure 2A–E).

Fig. 3. Interaction of HBXIP and survivin is regulated by phosphorylation. (A) HEK293 cells co-transfected with plasmids encoding FLAG–HBXIP and either wild-type (W) myc–survivin or myc–survivin (T34A) were immunoprecipitated with anti-myc antibody, followed by immunoblotting using anti-FLAG antibody (upper panel). Lysates were also blotted by anti-myc antibody (lower panel). (B) Endogenous survivin was immunoprecipitated from HeLa cells that had been transfected with control or kinase-dead Cdc2 mutant (DN) together with FLAG–HBXIP. Immunoprecipitates were analyzed by immunoblotting using anti-FLAG antibody (upper panel) or an antibody specific for phophorylated Thr34 survivin [(p)-SVV] (middle panel). Lysates were also blotted by anti-survivin antibody (lower panel). (C) In vitro binding experiments were performed, incubating either 0.25 µg of His6-survivin (left panel) or His6-T34E-survivin (right panel) with 1 µg of GST–CD40 (CTR) or GST–HBXIP immobilized on glutathione–Sepharose in 0.5 ml of binding buffer. Bound proteins were analyzed by immunoblotting using anti-survivin (upper panel) antisera. ‘Input’ represents 25 ng of His6-survivin or His6-T34E- survivin. GST-tagged proteins were also analyzed by immunoblotting using anti-GST antibody (lower panel).
HBXIP collaborates with survivin in suppressing caspase-9 activation
To explore the functional significance of interaction of HBXIP with survivin, we evaluated the effects of recombinant HBXIP alone and in combination with survivin on activation of caspases in cell lysates stimulated with Cyt-c. At concentrations <200 nM, adding either HBXIP or survivin to cell lysates only slightly suppressed generation of caspase protease activity, measured by hydrolysis of Ac-DEVD-AFC (Figure 4A). In contrast, combining HBXIP and survivin markedly suppressed Cyt-c-induced caspase activation, implying functional synergy. However, if survivin and HBXIP were added after Cyt-c, then caspase activity was not suppressed (data not shown), implying that these proteins block the activation event but do not suppress caspases once activated. Replacing either survivin or HBXIP with various control proteins (e.g. GST, GST–CD40, His6-TRAF3) failed to suppress caspase activity, confirming the specificity of the results (data not shown). In contrast to Cyt-c, the combination of survivin and HBXIP did not inhibit caspase activation induced in lysates by adding active caspase-8 or granzyme B, while XIAP did suppress it (Figure 4B and C). These findings suggest that survivin selectively inhibits caspase activation via the Cyt-c pathway, whereas XIAP has broader activity.
Fig. 4. HBXIP collaborates with survivin to suppress Cyt-c-induced caspase activation. Comparisons were made of effects of prior addition of purified HBXIP, survivin or the combination on caspase activity induced in cell extracts by Cyt-c (A), caspase-8 (B) or granzyme B (C), measuring AFC release from Ac-DEVD-AFC. Left panels show representative enzyme progress curves, indicating cumulative release of fluorophore from Ac-DEVD-AFC (RFU/µg). Right panels represent relative enzyme rates (RFU/min/µg) compared with samples treated with control protein (mean ± SD; n = 3 experiments). Several control (CTR) proteins were tested, with representative results shown, here using His6-TRAF3.
We explored the effects of HBXIP/survivin on Apaf1-mediated activation of pro-caspase-9, the apical protease in the Cyt-c pathway for apoptosis (Li et al., 1997), using purified proteins (Zou et al., 1999). After adding Cyt-c and dATP to induce Apaf1 oligomerization, caspase-9 activity was measured by cleavage of fluorigenic substrate Ac-LEHD-AFC (Figure 5A and B) and pro-caspase-9 processing was monitored by immunoblotting (Figure 5C). Neither survivin nor HBXIP was individually effective at blocking Apaf1-mediated activation and processing of pro-caspase-9 in vitro. In contrast, the combination of survivin and HBXIP effectively suppressed generation of caspase-9 protease activity (Figure 5A and B), and also reduced proteolytic processing of ∼50 kDa pro-caspase-9 into large (∼35 kDa) and small (∼12 kDa) subunits typical of the active protease (Figure 5C). Scanning densitometry analysis of the autoradiograms revealed a >80% reduction in the relative amount of large (∼35 kDa) subunit production when survivin and HBXIP were present (Figure 5C). Substituting various control proteins (e.g. GST, GST–CD40, His6-TRAF3) failed to inhibit Apaf1-induced activation of pro-caspase-9 (data not shown).

Fig. 5. Combination of HBXIP and survivin inhibits caspase-9 activation. Recombinant pro-caspase-9 was incubated with purified His6-Apaf1, Cyt-c and dATP, with or without recombinant HBXIP, survivin, or the combination of these proteins or various control (CTR) proteins. (A and B) Caspase-9 activity was measured by cleavage of fluorogenic substrate Ac-LEHD-AFC, or (C) proteolytic processing of ∼50 kDa pro-caspase-9 to large (∼35 kDa) and small (∼12 kDa) subunits was analyzed by immunoblotting analysis using anti-caspase-9 antibody. Data in (B) represent mean ± SD (n = 3), while data in (A) show representative enzyme progress curves. In (C), levels of recombinant proteins were also compared by immunoblotting using anti-HBXIP, anti-survivin and anti-His antibodies (lower three panels). Minus signs indicate addition of an equivalent amount of a control protein (GST–CD40 or His6-TRAF3) instead of survivin or HBXIP.
Survivin–HBXIP blocks recruitment of pro-caspase-9 to Apaf1
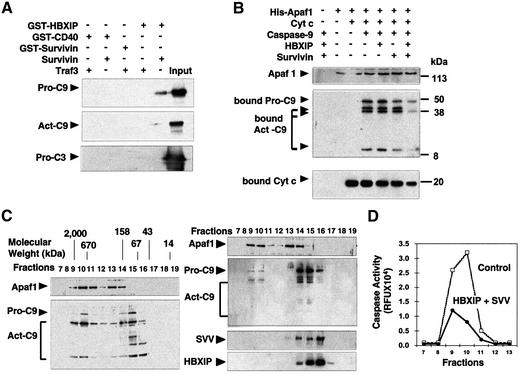
To explore how survivin and HBXIP suppress pro-caspase-9 activation, we tested whether these proteins can bind pro-Capase-9 in vitro. GST–HBXIP was incubated with His6-pro-caspase-9 in the presence or absence of purified survivin. HBXIP-bound proteins were analyzed by immunoblotting. For comparison, experiments were also performed using purified active caspase-3 and -9 (Stennicke et al., 1999). Without survivin, GST–HBXIP failed to ‘pull down’ caspases (Figure 6A). However, when survivin was included, then pro-caspase-9 was pulled down with GST–HBXIP, but not with control GST–CD40 protein. GST–HBXIP also pulled down active caspase-9, in the presence survivin, but much less efficiently than pro-caspase-9. No association with caspase-3 was found. When survivin was used alone (as a GST fusion protein) for pull-down assays, no binding to caspases was detected. Thus, HBXIP–survivin complexes can bind pro-caspase-9.
Fig. 6. Combination of HBXIP and survivin inhibits recruitment of pro-caspase-9 to activated Apaf1. (A) GST–survivin, GST–CD40 or GST–HBXIP with or without purified survivin (untagged) was incubated with His6-pro-caspase-9, active His6-caspase-9 (lacking CARD domain) or His6-pro-caspase-3. GST fusion proteins were recovered using glutathione–Sepharose and bound proteins were detected by immunoblotting using anti-caspase-9 or anti-caspase-3 antisera. An equivalent amount of proteins was loaded directly onto gels as a control (‘input’). (B) [35S]pro-caspase-9 was incubated with His6-Apaf1, Cyt-c and dATP, with or without GST–HBXIP and survivin. His6-Apaf1 and associated proteins were recovered by adsorption to Ni-resin, and bound proteins were analyzed by autoradiography (for caspase-9) or immunoblotting using anti-Apaf1 or anti-Cyt-c antibodies. (C) Purified His6-Apaf1, His6-pro-caspase-9, Cyt-c and dATP were incubated without (left panel) or with (right panel) purified survivin and HBXIP. Proteins were analyzed by gel-sieve chromatography using immunoblotting to detect proteins in eluted fractions. (D) Caspase activity in column fractions was determined by monitoring cleavage of Ac-DEVD-AFC after addition of pro-caspase-3.
Since HBXIP–survivin complexes bind pro-caspase-9, we tested whether these proteins interfere with binding of pro-caspase-9 to Apaf1. Accordingly, we incubated purified His6-Apaf1 with [35S]pro-caspase-9, Cyt-c and dATP, with or without survivin, HBXIP, or the combination of these proteins. Then, His6-Apaf1 was recovered on Ni-chelation resin, and associated proteins were analyzed by autoradiography (caspase-9) or by immunoblotting using anti-Cyt-c antibody. Without survivin and HBXIP, full-length ∼50 kDa pro-caspase-9 as well as the large (35–37 kDa) and small (∼12 kDa) subunits of processed caspase-9 were recovered with His6-Apaf1 on Ni-resin (Figure 6B). In contrast, when pro-caspase-9 was pre-incubated with HBXIP and survivin, the amount of pro-caspase and processed caspase-9 recovered with His6-Apaf1 on Ni-resin was significantly reduced, suggesting that HBXIP–survivin complexes prevented recruitment of pro-caspase-9 to activated Apaf1 (Figure 6B). When various control proteins were substituted for either survivin or HBXIP, then pro-caspase-9 association with Apaf1 was not blocked (data not shown). The combination of survivin and HBXIP also slightly reduced binding of Cyt-c to Apaf1 (Figure 6B), suggesting that the stability of this interaction may be reduced when pro-caspase-9 binding is blocked.
To further address the inhibitory mechanism of survivin–HBXIP, we analyzed apoptosome complexes by gel-filtration chromatography (Figure 6C). Without survivin and HBXIP, two peaks of Apaf1 were detected, and caspase-9 co-eluted with the larger of these complexes (Figure 6C, left panel), as expected (Zou et al., 1999). In contrast, when adding survivin and HBXIP, Apaf1 oligomerization occurred normally but very little caspase-9 co-eluted with oligomerized Apaf1. Also, more of the caspase-9 was present in the pro-enzyme form (∼50 kDa as opposed to ∼35 kDa). Moreover, column fractions in which the majority of the pro-caspase-9 eluted also contained survivin and HBXIP (Figure 6C, right panel). Monitoring fractions for caspase-9 activity revealed markedly reduced caspase-9 activity when survivin and HBXIP were included (Figure 6D). We conclude, therefore, that the combination of HBXIP and survivin interferes with apoptosome assembly, preventing recruitment of pro-caspase-9 to oligomerized Apaf1.
Survivin and HBXIP collaborate in suppressing apoptosis
We contrasted effects of transfecting plasmids encoding survivin, HBXIP or both of these proteins on apoptosis induced by staurosporine (STS) and anti-Fas antibody, stimuli that operate through caspase-9-dependent and -independent pathways, respectively (Salvesen, 2002). Also, wild-type HBXIP was compared with a mutant containing the first 61 amino acids of HBXIP protein, del-HBXIP(1–61), which we empirically determined to show reduced binding to survivin (Figure 7). At the plasmid concentrations used, either survivin or HBXIP only slightly suppressed apoptosis in transfected HT1080 cells (Figure 7, lower panel). In comparison, Bcl-XL [which inhibits Cyt-c release from mitochondria (Yang et al., 1997)] and CrmA [which inhibits caspase-8 (Zhou et al., 1997)] suppressed apoptosis induced by STS and anti-Fas antibody, respectively, serving as positive controls. When survivin and HBXIP were co-expressed in HT1080 cells, then apoptosis induced by STS (but not Fas) was suppressed (Figure 7). Immunoblot analysis of transfected cells confirmed production of all proteins and demonstrated comparable levels of HBXIP and del-HBXIP(1–61) (data not shown). Together, these data demonstrate collaboration of survivin and HBXIP in selectively suppressing the caspase-9-dependent pathway for apoptosis, consistent with our in vitro data that these proteins block pro-caspase-9 activation.

Fig. 7. HBXIP/survivin selectively suppresses caspase-9-dependent apoptosis. Upper panel: full-length HBXIP and HBXIP(1–61) proteins were produced as GST fusions, purified and then tested for binding to His6-survivin or control protein (His6-TRAF3). Bound proteins were analyzed by immunoblotting using anti-GST antisera (upper panel). An equivalent amount of GST fusion protein was loaded directly in gels as a control (‘input’). Lower panel: HT1080 cells were transiently transfected with expression plasmids encoding myc–survivin, FLAG–HBXIP, FLAG–HBXIP(1–61), Bcl-XL (for STS) or CrmA (for anti-Fas), alone or in various combinations, with pEGFP marker plasmid, using pcDNA3 to normalize total DNA content. Then cells were cultured with STS or anti-Fas antibody, and the percentage of apoptotic cells was determined by DAPI staining (mean ± SD; n = 3) a day later.
Caspase regulation by endogenous HBXIP and survivin
To explore the role of HBXIP in the pathogenesis of human liver disease associated with HBV infection, we first analyzed the expression of this protein by immunoblotting in primary hepatocellular carcinoma tissues, as well as non-cancerous regions of the same livers of patients with chronic HBV infection. As a control for non-HBV infection, we also examined normal liver tissue from patients with metastatic colon cancer. Levels of HBXIP protein were elevated 2- to 7-fold in both cancerous and non-cancerous liver tissue of patients with chronic HBV infection compared with hepatic tissue without HBV infection (Figure 8A), based on scanning densitometry analysis of immunoblots and normalization to α-tubulin. Probing the same blots with anti-survivin antibody revealed elevated survivin protein in tumor tissue compared with non-cancerous regions of the same livers (Figure 8A).

Fig. 8. Elevations of survivin and HBXIP associated with caspase suppression. (A) Protein samples from tumors (T) and non-malignant tissue (NT) of three patients with HBV-related hepatocellular carcinoma and two normal livers specimens (N1 and N2) were normalized for protein content, and analyzed by SDS–PAGE/immunoblotting using antisera specific for HBXIP, survivin or α-tubulin. (B) Caspase activity in the same tissue samples was measured using the fluorigenic substrate Ac-DEVD-AFC after addition of Cyt-c and dATP (left panel) or granzyme B (right panel). Results are expressed relative to caspase activity generated in the normal liver specimen, N1 (mean ± SD; n = 3).
Since the combination of survivin and HBXIP interferes with Cyt-c-induced activation of pro-caspase-9, we tested whether a correlation exists between elevated expression of these proteins in HBV-associated hepatocellular carcinomas and resistance to Cyt-c-induced caspase activation. Accordingly, lysates from liver tissues were normalized for total protein content, then incubated with Cyt-c and dATP to induce Apaf1-mediated activation of caspases. Compared with non-infected liver tissue, Cyt-c-inducible caspase activity was significantly reduced in lysates of both cancerous (P < 0.01) and non-cancerous (P < 0.01) liver tissue from patients with chronic HBV infection (Figure 8B, left panel), with induction of DEVD-cleaving caspase activity more depressed in cancerous tissues than non-cancerous tissues from HBV-infected individuals (P < 0.01). In contrast, no significant difference was found in caspase activity induced by exogenously added granzyme B (Figure 8B, right panel). Thus, HBV-associated elevations in expression of HBXIP correlate with selective resistance to Cyt-c in extracts from patient tissues.
To determine whether HBXIP is necessary for the inhibitory effect of survivin, we used small-interfering RNA (siRNA) to reduce expression of endogenous HBXIP in HeLa cells. Transfection of HBXIP-specific but not control double-strand synthetic RNAs reduced endogenous HBXIP protein levels in HeLa cells, which was sustained for at least 3 days (Figure 9A). We also confirmed that the levels of other proteins required for Cyt-c responsiveness (Apaf1, pro-caspase-9, pro-caspase-3) were unaffected by HBXIP–siRNA (data not shown), and that levels of endogenous survivin were unaltered (Figure 9A). Cyt-c was more effective at activating caspases in cell extracts treated with HBXIP–siRNA compared with control RNA-treated cells (Figure 9B), confirming a role for HBXIP as an endogenous antagonist of this caspase activation pathway. In control extracts, adding purified survivin suppressed Cyt-c-induced activation of caspases. In contrast, survivin failed to suppress caspase activation in extracts in which HBXIP expression was knocked down (Figure 9B). By comparison, siRNA-mediated knock-down of HBXIP did not affect the inhibitory effect of XIAP on caspase activation (Figure 9B), demonstrating the specificity of the results. Similar conclusions were reached from experiments using intact cells in which endogenous HBXIP expression was knocked down by siRNA, showing that more apoptosis was induced by agents that trigger caspase-9-dependent apoptosis when HBXIP levels were reduced (Figure 9C).

Fig. 9. Regulation of caspase activation and apoptosis by endogenous HBXIP. (A) HeLa cells were transfected with siRNA targeting HBXIP or control RNA (CTR), and cells were analyzed by immunoblotting, using anti-HBXIP (upper panel), anti-survivin (middle) and anti-α- tubulin (lower) antisera, thus verifying siRNA-mediated reductions in endogenous HBXIP expression. (B) Extracts prepared from HeLa cells after treatment with HBXIP–siRNA or control RNA were incubated with Cyt-c and dATP, in the presence or absence of recombinant survivin (left panel) or XIAP (right panel), and caspase activity was measured based on release of AFC from Ac-DEVD-AFC substrate [mean ± SD; n = 3). (C) The percentage of apoptotic cells (mean + SD; n = 3) was determined by DAPI staining following culture of control RNA- or HBXIP siRNA-transfected HeLa cells with 25 µM VP-16 or STS.
HBX protein collaborates with HBXIP in suppressing caspase activation
Viral oncoprotein HBX has been implicated in hepatocellular carcinogenesis (reviewed in Murakami, 2001). HBX association with HBXIP was confirmed using bacteria-expressed HBXIP and in vitro translated His6-HBX protein (Figure 10A) [attempts to produce soluble HBX in bacteria failed (data not shown)]. HBX, in contrast, did not bind survivin. Next, since HBX associates with HBXIP, we tested whether this viral protein effects caspases. Adding HBX protein to lysates prepared from HepG2 hepatocellular cancer cells, which contain elevated endogenous survivin (data not shown), suppressed Cyt-c-induced caspase activation. In contrast, control proteins prepared in the same manner had little effect (Figure 10B). Also, HBX protein increased the effects of HBXIP and survivin on caspase suppression (Figure 10B).
Fig. 10. The X protein of the hepatitis B virus (HBX) associates with survivin through HBXIP and suppresses caspase activation. (A) In vitro protein binding assays were performed using His6-HBX incubated with GST–HBXIP, GST–survivin or GST–CD40 (control) immobilized on glutathione–Sepharose. Bound proteins were analyzed by immunoblotting using anti-HBX antibody. (B) His6-HBX or His6-Traf3 (CTR) proteins, purified His6-HBXIP, purified survivin or various combinations of these proteins were added to 293 cell extracts, normalizing all samples for total protein added using control recombinant proteins. Cyt-c and dATP were then added, and caspase activity was measured 0.5 h later, based on hydrolysis of Ac-DEVD-AFC, and compared with samples treated with control proteins (mean ± SD; n = 3). (C) HEK293 cells were transiently transfected with plasmids encoding FLAG-tagged-HBX or FLAG–SIP (as a control) together with Myc-survivin or HA–HBXIP or control plasmid. Lysates were subjected to immunoprecipitation using anti-FLAG antibody, demonstrating increased association of survivin with HBX when HBXIP was co-expressed (compare the last two lanes on the right). Immunoprecipitates were analyzed by immunoblotting using anti-survivin antibody (upper panel). Lysates were also analyzed by immunoblotting using anti-HBX (middle panel) or anti-HA antibodies (lower panel), confirming protein production. (D) His6-pro-caspase-9 was incubated with GST–HBXIP(+) or GST–CD40 control protein (–), with or without His6-HBX and untagged survivin. GST fusion proteins were recovered on glutathione–Sepharose and bound proteins were detected by immunoblotting using anti-caspase-9, anti-survivin or anti-HBX antisera. (E) HepG2 cell extracts were immunodepleted using anti-survivin antisera or preimmune serum (CTR), and equivalent amounts were analyzed by immunoblotting using anti-survivin antibody (top panel). Then equivalent volumes of extracts were analyzed for caspase activity based on Ac-DEVD-AFC cleavage, where lysates were incubated with HBX (+) or control protein (–) prior to adding Cyt-c and dATP.
To examine whether HBX protein associates with HBXIP–survivin complexes, co-immunoprecipitation assays were performed using epitope-tagged HBX, HBXIP and survivin expressed by transient transfection in HEK293T cells. Since HBX does not directly bind survivin (Figure 10A), we reasoned that overexpressing HBXIP would increase the amounts of survivin immunoprecipitated with HBX by bridging these two proteins together. Indeed, when HBX and survivin were co-expressed with HBXIP, more survivin was associated with HBX immunoprecipitates (Figure 10C). Immunoblot analysis of lysates derived from the transfected cells confirmed production of equivalent amounts of survivin and HBXIP proteins, regardless of whether HBX was co-expressed (data not shown). Thus, HBX, HBXIP and survivin form complexes.
To determine the effects of HBX on binding of survivin–HBXIP complexes to pro-caspase-9, we performed protein interaction assays, examining association of pro-caspase-9 with GST–HBXIP when survivin, HBX or both proteins were added (Figure 10D). GST–HBXIP pulled down pro-caspase-9 when survivin was included in the binding assays. Addition of HBX did not impair pro-caspase-9 association with GST–HBXIP, and instead increased pro-caspase-9 binding slightly.
To explore whether HBX suppresses caspase activity through a survivin-dependent mechanism, we immunodepleted endogenous survivin from HepG2 cell lysates and then tested the effect of adding HBX protein on Cyt-c-induced caspase activation (Figure 10E). In extracts subjected to mock immunodepletion, HBX suppressed caspase activation by approximately half. In contrast, when survivin was immunodepleted, Cyt-c was more potent at activating caspases and adding HBX had little inhibitory effect. Thus, HBX fails to inhibit caspases in the absence of survivin.
Discussion
Survivin normally functions as an essential factor for chromosome segregation and cytokinesis during cell division. However, when overexpressed, survivin accumulates in the cytosol, where it assumes an anti-apoptotic function. This anti-apoptotic function of survivin may only be relevant to situations of overexpression, representing a pathological rather than a physiological function of this protein. Devising strategies for nullifying survivin in cancers has been hampered by a lack of knowledge about its biochemical mechanism. Unlike IAP family members, such as XIAP (Deveraux et al., 1997; Deveraux and Reed, 1999; Riedl et al., 2001), a role for survivin as a direct inhibitor of caspases has been controversial, based on studies using recombinant proteins in vitro (Banks et al., 2000; Verdecia et al., 2000; Shin et al., 2001). We show here that survivin differs markedly from XIAP in its mechanism of caspase inhibition. Unlike XIAP, which can inhibit caspases after activation, survivin is only effective if added before stimulating caspase activation. Also, unlike XIAP, which can inhibit caspases in cell extracts stimulated with Cyt-c, caspase-8 or granzyme B, survivin is selective for Cyt-c.
Since survivin demonstrated caspase inhibitory activity in cell extracts but not when using the purified protein, we suspected that survivin requires a partner protein, and identified HBXIP as a candidate. Consistent with the hypothesis, survivin lost its ability to suppress Cyt-c-induced caspase activation in cell extracts and to inhibit apoptosis when expressed in cells in which endogenous HBXIP was reduced by siRNA. In contrast, XIAP retained caspase-inhibitory activity. Furthermore, in contrast to survivin, which together with HBXIP precluded pro-caspase-9 recruitment to activated Apaf1, XIAP reportedly associates with Apaf1–caspase-9 complexes (apoptosomes) and does not interfere with caspase-9 interaction with Apaf1 (Bratton et al., 2001). Thus, while both survivin and XIAP target caspase-9, they utilize different mechanisms to oppose this protease.
HBXIP was originally isolated as a human protein which binds viral HBX protein (Melegari et al., 1998). Expression of HBXIP mRNA occurs in nearly all tissues, and is not limited to liver (Melegari et al., 1998). Little is known about the physiological roles of HBXIP, but it is found in mice and other rodents, suggesting an evolutionarily conserved function. We propose that HBXIP is an anti-apoptotic cofactor of survivin. Consistent with this idea, siRNA-mediated reductions in endogenous HBXIP levels sensitized cancer cells to apoptosis, while overexpression of HBXIP suppressed apoptosis in collaboration with survivin. However, since the anti-apoptotic function of survivin may be manifest only when overexpressed in cancer, it is possible that HBXIP also interacts additionally with other unidentified members of the IAP/BIR family and/or that it participates in the cell cycle actions of survivin. Interestingly, consistent with previous reports indicating a role for phosphorylation of survivin on Thr34 for its anti-apoptotic activity (O’Connor et al., 2000), association of HBXIP with survivin was abrogated either by mutation of Thr34 to a non-phosphorylatable residue, or by reducing phosphorylation of endogenous survivin using Cdc2 dominant-negative. Conversely, purified HBXIP bound survivin T34E with higher affinity than wild-type survivin.
Increased HBXIP was found in both cancerous and non-malignant liver tissue of humans with chronic HBV infection, compared with normal hepatic tissue. In this regard, chronic HBV infection is known to cause pre-neoplastic changes in liver (Lok, 2000), perhaps explaining elevations in HBXIP in both non-malignant and tumor tissue of HBV-diseased livers. Although caspase activity studies using lysates from clinical specimens demonstrated a correlation between elevated HBXIP and reduced sensitivity to Cyt-c, further work is needed to determine the frequency of HBXIP overexpression in hepatocellular carcinoma and its functional importance for apoptosis resistance.
More than 380 million HBV carriers are present worldwide today, with chronic HBV infection representing a major global pathogenic factor for development of hepatocellular carcinoma (Lok, 2000). A crucial role of HBV in hepatocarcinogenesis is beyond doubt, while the mechanisms by which HBV causes transformation of hepatocytes remain unclear. The HBV genome comprises a 3.2 kb circular double-stranded DNA molecule with overlapping open reading frames (ORFs) encoding four viral proteins, including HBX, a 154-amino-acid protein that has no recognizable counterpart in humans or other mammalian species. HBX is essential for replication of woodchuck HBV, and transgenic mice expressing HBX have increased incidence of hepatocellular cancer when exposed to chemical carcinogens (Kim et al., 1991). Thus, HBX is a candidate transforming gene of HBV. Like many viral oncoproteins, HBX is a multifunctional protein. HBX exhibits effects on gene transcription, cell proliferation and apoptosis, and has multiple putative cellular targets besides HBXIP (reviewed in Murakami, 2001). The effects of HBX on apoptosis are controversial, with evidence suggestive either of suppression or promotion of apoptosis, depending on cellular context and stimulus. Interestingly, among the reported molecular effects of HBX is transcription-independent suppression of caspases (Gottlob et al., 1998), although many alternative mechanisms have also been proposed (reviewed in Murakami, 2001). Our data indicate that HBX can associate indirectly with survivin, through HBXIP. Moreover, depletion of survivin from cell extracts abolishes the ability of HBX to suppress caspase activation in vitro. Thus, our data are consistent with the hypothesis that HBX modulates apoptosis pathways through interactions with HBXIP– survivin complexes. Effects of HBXIP and HBX on other facets of survivin function remain to be addressed, particularly the role of survivin as a regulator of cell division.
Materials and methods
Yeast assays
A Jurkat T-cell cDNA library was screened by the yeast two-hybrid method (Matsuzawa and Reed, 2001), using pGilda-survivin, which produces fusion proteins with a LexA DNA-binding domain. Specificity of interactions was confirmed by mating experiments using a panel of yeast containing various control bait plasmids (pGilda-Bax, -Fas, -caspase-9), and by re-transformation experiments (see Supplementary figure 2).
cDNA cloning and plasmid construction
A cDNA encoding human HBXIP was generated by reverse transcription from Jurkat T-cell mRNA using SuperScript II (Gibco, Rockville, MA), followed by amplification using the Expand High Fidelity PCR system (Roche, Mannheim, Germany) and oligonucleotide primers as follows: 5′-GACGAATTCATGGAGGCGACCTTGGAGCA-3′ (forward) and 5′-GATCTCGAGTCAAGAGGCCATTTTGTGCA-3′ (reverse). The resultant cDNA fragments were ligated into pcDNA3-FLAG for mammalian expression (Matsuzawa and Reed, 2001), or into pET21d-N-His6 and pGEX4T-1 for expression in bacteria. Various fragments of survivin cDNA were also PCR amplified from pcDNA3-survivin (Tamm et al., 1998) and subcloned into pcDNA3 (Invitrogen, Carlsbad, CA). Survivin (T34E and T34A) mutants were constructed by PCR. The gene encoding HBX was synthesized by PCR from DNA obtained from patients with hepatocellular carcinoma (Marusawa et al., 2000).
Antibodies
Polyclonal antisera were generated in rabbits and chickens using purified recombinant His6-HBXIP as an immunogen. Rabbit polyclonal antibodies against pro-caspase-9, pro-caspase-3, XIAP and survivin have been described (Tamm et al., 1998). Phospho-specific antibody recognizing phosphorylated survivin was obtained from D.Altieri (University of Massachusetts, MA) (O’Connor et al., 2000). Rabbit anti-Apaf1 antibody was purchased from Cayman (Ann Arbor, MI). Mouse monoclonal antibodies against active caspase-9 and Cyt-c were purchased from PharMingen (San Diego, CA). Rabbit polyclonal anti-HBX antibody was a gift of R.J.Schneider (New York University, NY).
Proteins production and binding assays
Recombinant proteins were purified as described (Deveraux and Reed, 1999; Zou et al., 1999). His6-HBX protein was made using the Rapid Translation System (Roche), using 2 µl of reaction product for caspase assays. Purified GST fusion protein or His6-tagged proteins immobilized on glutathione–Sepharose beads or nickel beads, respectively, were incubated in 1% Triton X-100/phosphate-buffered saline (PBS) for 1 h at 4°C. Beads were washed with binding buffer (5 mM MgCl2, 10% glycerol, 0.5 mg/ml BSA, 5 mM 2-mercaptoethanol and 50 mM Tris–HCl pH 7.5) and incubated overnight at 4°C with various recombinant proteins or in vitro translated 35S-labeled proteins produced using TNT-coupled reticulocyte lysates (Promega, Madison, WI). Protein on beads were washed four times in binding buffer, and bound proteins were eluted in SDS sample buffer and subjected to SDS–PAGE, as described (Deveraux and Reed, 1999).
Caspase assays
Primary hepatocellular carcinomas and non-cancerous liver tissue were obtained from patients undergoing biopsy or surgery at Kyoto University Hospital after obtaining informed consent. Patients with chronic HBV infection were confirmed seropositive for HBs antigen. Cytosolic extracts from cells or liver tissues were prepared in buffer A (10 mM KCl, 1.5 mM MgCl2, 1 mM EDTA, 1 mM EGTA, 1 mM dithiothreitol, 0.1 mM PMSF and 20 mM HEPES–KOH pH 7.4), as described (Deveraux et al., 1997). After measuring protein levels (Biorad, Hercules, CA), 50 µg of protein sample was incubated with either 10 nM recombinant caspase-8, 1 ng of granzyme B (Calbiochem, San Diego, CA) or 10 µM Cyt-c and 1 mM dATP, with or without various purified proteins in caspase buffer (1 mM EDTA, 0.1% CHAPS, 10% sucrose and 25 mM HEPES pH 7.2). Then, 5 µl reaction mixtures were incubated with fluorogenic caspase substrate Ac-DEVD-AFC (Calbiochem) in 100 µl of caspase buffer. Caspase activity was measured using a LS50B fluorometric plate reader (Perkin-Elmer, Norwalk, CT) in the kinetic mode with excitation and emission wavelengths of 405 and 510 nm, respectively. Release of AFC from substrate peptide was compared after 0.5 h.
Apoptosome experiments
Apoptosome activity was assayed as described using proteins produced in and purified from baculovirus-infected insect cells (Zou et al., 1999). Recombinant pro-caspase-9 (4 nM) was incubated with purified survivin (40 nM), HBXIP (40 nM), the combination of these proteins, or equivalent amounts of various control proteins such as GST–CD40 or His6-TRAF3 at 30°C for 10 min. Then, 4 nM recombinant Apaf1 was added, followed by 200 µM dATP, 0.6 µM Cyt-c and 10 µM recombinant pro-caspase-3 in 40 µl (total) of buffer A. After incubation, 160 µl of caspase buffer containing Ac-DEVD-AFC (100 µM final) were added. Alternatively, for measurements of caspase-9 activity, 0.2 µM pro-caspase-9 was incubated with 100 nM Apaf1 with a 2 µM concentration of various recombinant proteins under the same conditions, then Ac-Leu-Glu-His-Asp-AFC (Ac-LEHD-AFC) (Calbiochem) was added.
Gel-filtration chromatography
Recombinant pro-caspase-9 (2 µg) was incubated with or without 5 µg of survivin and 5 µg of HBXIP at 30°C for 0.5 h in 20 µl of buffer A. Then, 2 µg of Apaf1, 200 µM dATP and 0.6 µM Cyt-c were added (total volume 55 µl). After incubation at 30°C for 0.5 h, proteins were passed through a Superdex-200 column (Amersham Biosciences, Piscataway, NJ), collecting 100 µl fractions, and 20 µl of each fraction were subjected to SDS–PAGE, then blotted with anti-HBXIP, anti-survivin, anti-Apaf1 and anti-caspase-9 antibodies. Alternatively, fractions were incubated with GST–HBXIP, and proteins were recovered on glutathione–Sepharose.
Co-immunoprecipitation and immunodepletion assays
Cells were lysed in IP buffer (0.5% NP-40, 1 mM EDTA, 135 mM NaCl and 20 mM Tris–HCl pH 7.5) containing protease inhibitors (Complete; Roche), and then incubated with primary antibody immobilized on protein G–Sepharose 4B (Zymed, San Francisco, CA) at 4°C overnight with constant rotation. Immunoprecipitates were washed with IP buffer four times and suspended in SDS sample buffer, then boiled and analyzed by SDS–PAGE/immunoblotting. Immunodepletion analysis was performed using 5 µl of anti-survivin antiserum or preimmune serum conjugated to 20 µl of protein A–Sepharose.
Subcellular fractionation
Cells were lysed in hypotonic buffer [10 mM Tris–HCl pH 7.5, 10 mM NaCl, 1 mM EDTA, Complete protease inhibitors (Roche)] and centrifuged at 700 g for 5 min to obtain nuclear pellets. The resulting supernatants were further centrifuged at 100 000 g for 60 min to obtain membranes (pellet) and soluble cytosolic (supernatant) fractions.
Apoptosis assays
Cells were cultured in DMEM with 10% fetal calf serum, 1 mM l-glutamine and antibiotics. Cells were transfected with various plasmids in combination with pEGFP (Clontech) using Fugene-6 transfection reagent (Roche). After culturing for 1.5 days, apoptosis was induced by anti-Fas antibody CH-11 (500 ng/ml; MBL, Nagoya, Japan), or STS (100 nM). Both floating and attached cells were collected 24 h after apoptosis induction, and analyzed by 1 µg/ml 4′,6-diamidino-2′-phenylindole dihydrochloride (DAPI) staining for assessing nuclear morphology, counting the percentage of apoptotic cells by fluorescence microscopy among ≥200 GFP-positive cells.
siRNA
siRNA duplexes composed of 21-nucleotide sense and antisense strands were synthesized by Dharmacon Research (Lafayette, CO). RNA oligonucleotides used for targeting HBXIP were 5′-GCAGCUAAGCUAACCUCUGTT-3′ (sense) and 5′-TTCGUCGAUUCGAUUGGAGAC-3′ (antisense). Control siRNA contained six nucleotide mismatches. HeLa cells (2.5 × 105) were plated in 6 cm wells 24 h before transfection. siRNA (20 µM) in 25 µl of oligofectamine reagent (Invitrogen) was incubated in serum-free Opti-MEM medium for 20 min, then the transfection mixture was added to cells, incubated at 37°C for 4 h, followed by addition of fresh medium containing serum. At 1.5 days post-transfection, cells were analyzed for apoptosis or lysed for immunoblotting.
Supplementary data
Supplementary data are available at The EMBO Journal Online.
Acknowledgments
Acknowledgements
We would like to thank R.J.Schneider for HBX antibody, D.Altieri for anti-phospho-survivin antibody, G.Salvesen for caspase-9, K.Okada for plasmids, S.Krajewski for assistance with HBXIP antibody, M.Krajewska for immunostaining, T.Miyazaki and T.Kawai for helpful advice, R.Cornell for manuscript preparation, and the National Institutes of Health (NIH) for their support (AG15393-05).
References
- Ambrosini G., Adida,C. and Altieri,D.C. (1997) A novel anti-apoptosis gene, Survivin, expressed in cancer and lymphoma. Nat. Med., 3, 917–921. [DOI] [PubMed] [Google Scholar]
- Banks D.P., Plescia,J., Altieri,D.C., Chen,J., Rosenberg,S.H., Zhang,H. and Ng,S.C. (2000) Survivin does not inhibit caspase-3 activity. Blood, 96, 4002–4003. [PubMed] [Google Scholar]
- Bratton S.B., Walker,G., Srinivasula,S.M., Sun,X.M., Butterworth,M., Alnemri,E.S. and Cohen,G.M. (2001) Recruitment, activation and retention of caspases-9 and -3 by Apaf-1 apoptosome and associated XIAP complexes. EMBO J., 20, 998–1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cryns V. and Yuan,Y. (1998) Proteases to die for. Genes Dev., 12, 1551–1570. [DOI] [PubMed] [Google Scholar]
- Deveraux Q.L. and Reed,J.C. (1999) IAP family proteins: suppressors of apoptosis. Genes Dev., 13, 239–252. [DOI] [PubMed] [Google Scholar]
- Deveraux Q.L., Takahashi,R., Salvesen,G.S. and Reed,J.C. (1997) X-linked IAP is a direct inhibitor of cell death proteases. Nature, 388, 300–304. [DOI] [PubMed] [Google Scholar]
- Gottlob K., Fulco,M., Levrero,M. and Graessmann,A. (1998) The hepatitis B virus HBx protein inhibits caspase 3 activity. J. Biol. Chem., 273, 33347–33353. [DOI] [PubMed] [Google Scholar]
- Grossman D., Kim,P.J., Blanc-Brude,O.P., Brash,D.E., Tognin,S., Marchisio,P.C. and Altieri,D.C. (2001) Transgenic expression of survivin in keratinocytes counteracts UVB-induced apoptosis and cooperates with loss of p53. J. Clin. Invest., 108, 991–999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim C.M., Koike,K., Saito,I., Miyamura,T. and Jay,G. (1991) HBx gene of hepatitis B virus induces liver cancer in transgenic mice. Nature, 351, 317–320. [DOI] [PubMed] [Google Scholar]
- Li F., Ambrosini,G., Chu,E.Y., Plescia,J., Tognin,S., Marchisio,P.C. and Altieri,D.C. (1998) Control of apoptosis and mitotic spindle checkpoint by survivin. Nature, 396, 580–587. [DOI] [PubMed] [Google Scholar]
- Li P., Nijhawan,D., Budihardjo,I., Srinivasula,S.M., Ahmad,M., Alnemri,E.S. and Wang,X. (1997) Cytochrome c and dATP-dependent formation of Apaf-1/Caspase-9 complex initiates an apoptotic protease cascade. Cell, 91, 479–489. [DOI] [PubMed] [Google Scholar]
- Lok A.S. (2000) Hepatitis B infection: pathogenesis and management. J. Hepatol., 32, 89–97. [DOI] [PubMed] [Google Scholar]
- Marusawa H., Uemoto,S., Hijikata,M., Ueda,Y., Tanaka,K., Shimotohno,K. and Chiba,T. (2000) Latent hepatitis B virus infection in healthy individuals with antibodies to hepatitis B core antigen. Hepatology, 31, 488–495. [DOI] [PubMed] [Google Scholar]
- Matsuzawa S. and Reed,J.C. (2001) Siah-1, SIP, and Ebi collaborate in a novel pathway for β-catenin degradation linked to p53 responses. Mol. Cell, 7, 915–926. [DOI] [PubMed] [Google Scholar]
- Melegari M., Scaglioni,P.P. and Wands,J.R. (1998) Cloning and characterization of a novel hepatitis B virus x binding protein that inhibits viral replication. J. Virol., 72, 1737–1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakami S. (2001) Hepatitis B virus X protein: a multifunctional viral regulator. J. Gastroenterol., 36, 651–660. [DOI] [PubMed] [Google Scholar]
- O’Connor D.S., Grossman,D., Li,F., Plescia,J., Zhang,H., Villa,A., Tognin,S., Marchisio,P.C. and Altieri,D.C. (2000) Regulation of apoptosis at cell division by p34cdc2 phosphorylation of survivin. Proc. Natl Acad. Sci. USA, 97, 13103–13107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed J.C. (2001) The survivin saga goes in vivo. J. Clin. Invest., 108, 965–969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed J.C. and Bischoff,J.R. (2000) BIRinging chromosomes through cell division—and survivin the experience. Cell, 102, 545–548. [DOI] [PubMed] [Google Scholar]
- Riedl S.J., Renatus,M., Schwarzenbacher,R., Zhou,Q., Sun,C., Fesik,S.W., Liddington,R.C. and Salvesen,G.S. (2001) Structural basis for the inhibition of caspase-3 by XIAP. Cell, 104, 791–800. [DOI] [PubMed] [Google Scholar]
- Salvesen G.S. (2002) Caspases: opening the boxes and interpreting the arrows. Cell Death Differ., 9, 3–5. [DOI] [PubMed] [Google Scholar]
- Shin S., Sung,B.-J., Cho,Y.-S., Kim,H.-J., Ha,N.-C., Hwang,J.-I., Chung,C.-W., Jung,Y.-K. and Oh,B.-H. (2001) An anti-apoptotic protein human survivin is a direct inhibitor of caspase-3 and -7. Biochemistry, 40, 1117–1123. [DOI] [PubMed] [Google Scholar]
- Stennicke H.R., Deveraux,Q.L., Humke,E.W., Reed,J.C., Dixit,V.M. and Salvesen,G.S. (1999) Caspase-9 can be activated without proteolytic processing. J. Biol. Chem., 274, 8359–8362. [DOI] [PubMed] [Google Scholar]
- Tamm I., Wang,Y., Sausville,E., Scudiero,D.A., Oltersdorf,V.N. and Reed,J.C. (1998) IAP-family protein survivin inhibits caspase activity and apoptosis induced by Fas (CD95), Bax, and anticancer drugs. Cancer Res., 58, 5315–5320. [PubMed] [Google Scholar]
- Velculescu V.E. et al. (1999) Analysis of human transcriptomes. Nat. Genet., 23, 387–388. [DOI] [PubMed] [Google Scholar]
- Verdecia M.A., Huang,H., Dutil,E., Kaiser,D.A., Hunter,T. and Noel,J.P. (2000) Structure of the human anti-apoptotic protein survivin reveals a dimeric arrangement. Nat. Struct. Biol., 7, 602–608. [DOI] [PubMed] [Google Scholar]
- Yang J., Liu,X., Bhalla,K., Kim,C.N., Ibrado,A.M., Cai,J., Peng,I.-I., Jones,D.P. and Wang,X. (1997) Prevention of apoptosis by Bcl-2: release of cytochrome c from mitochondria blocked. Science, 275, 1129–1132. [DOI] [PubMed] [Google Scholar]
- Zhou Q., Snipas,S., Orth,K., Muzio,M., Dixit,V.M. and Salvesen,G.S. (1997) Target protease specificity of the viral serpin CrmA: analysis of five caspases. J. Biol. Chem., 272, 7797–7800. [DOI] [PubMed] [Google Scholar]
- Zou H., Li,Y., Liu,X. and Wang,X. (1999) An APAF-1 cytochrome c multimeric complex is a functional apoptosome that activates procaspase-9. J. Biol. Chem., 274, 11549–11556. [DOI] [PubMed] [Google Scholar]