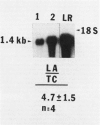
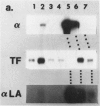
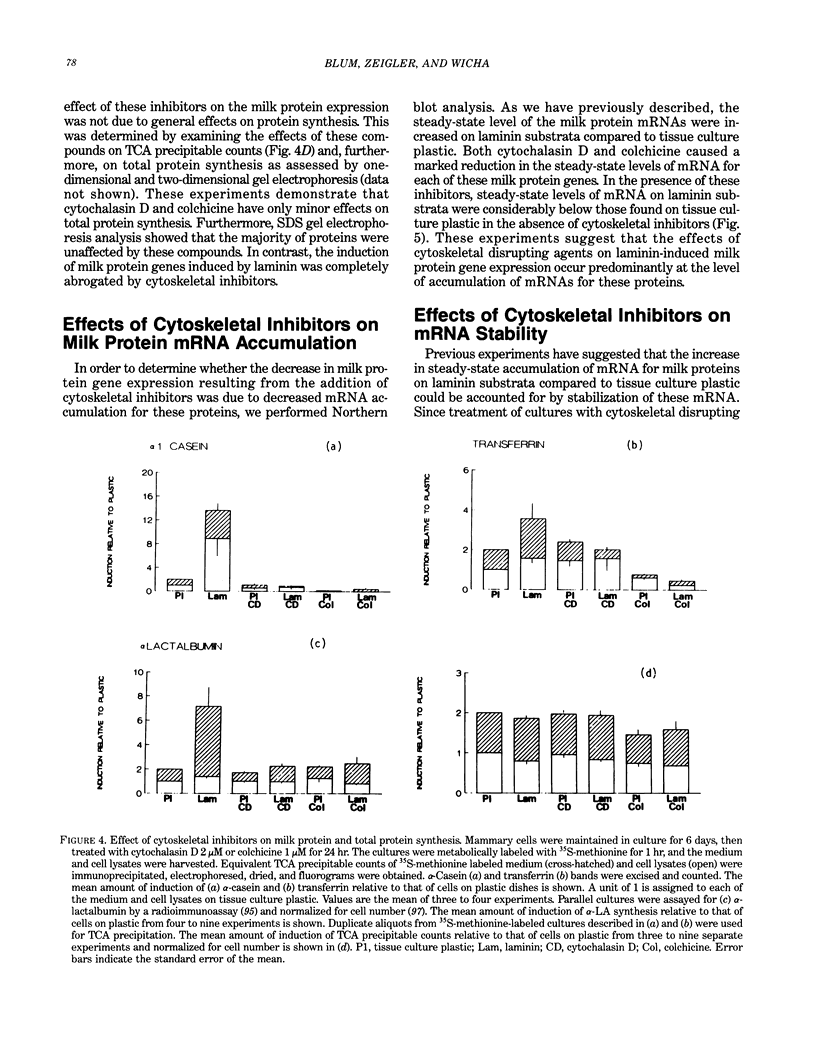
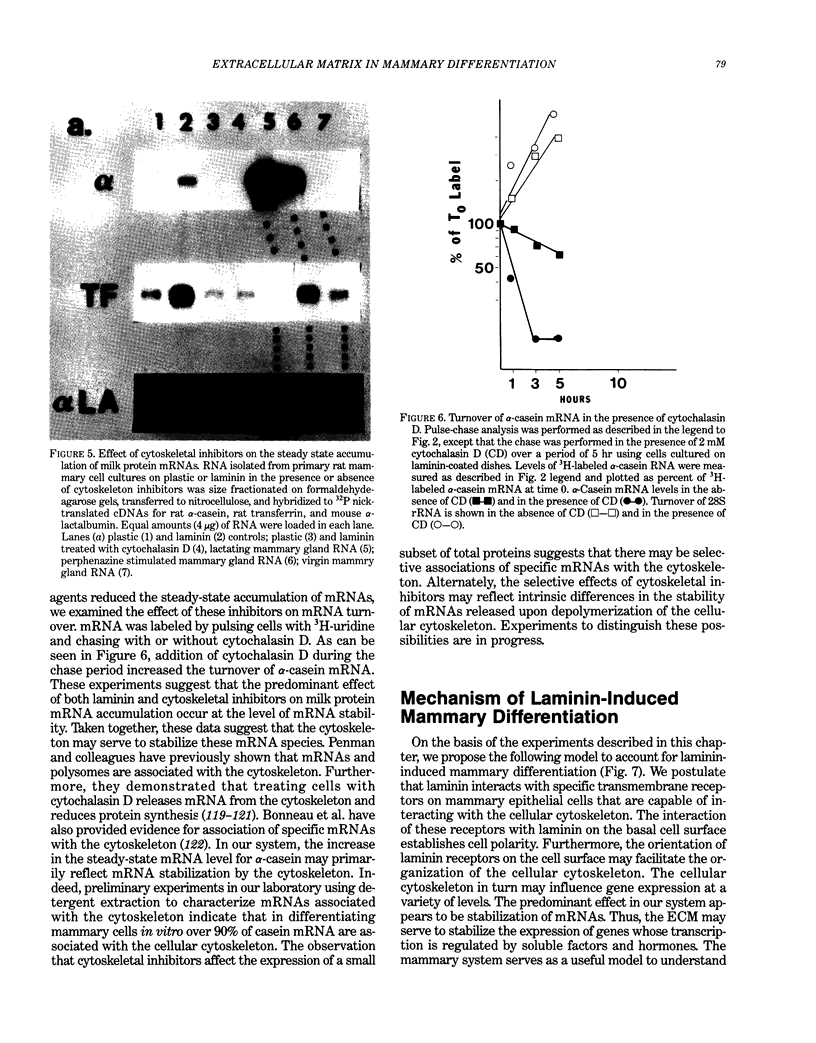
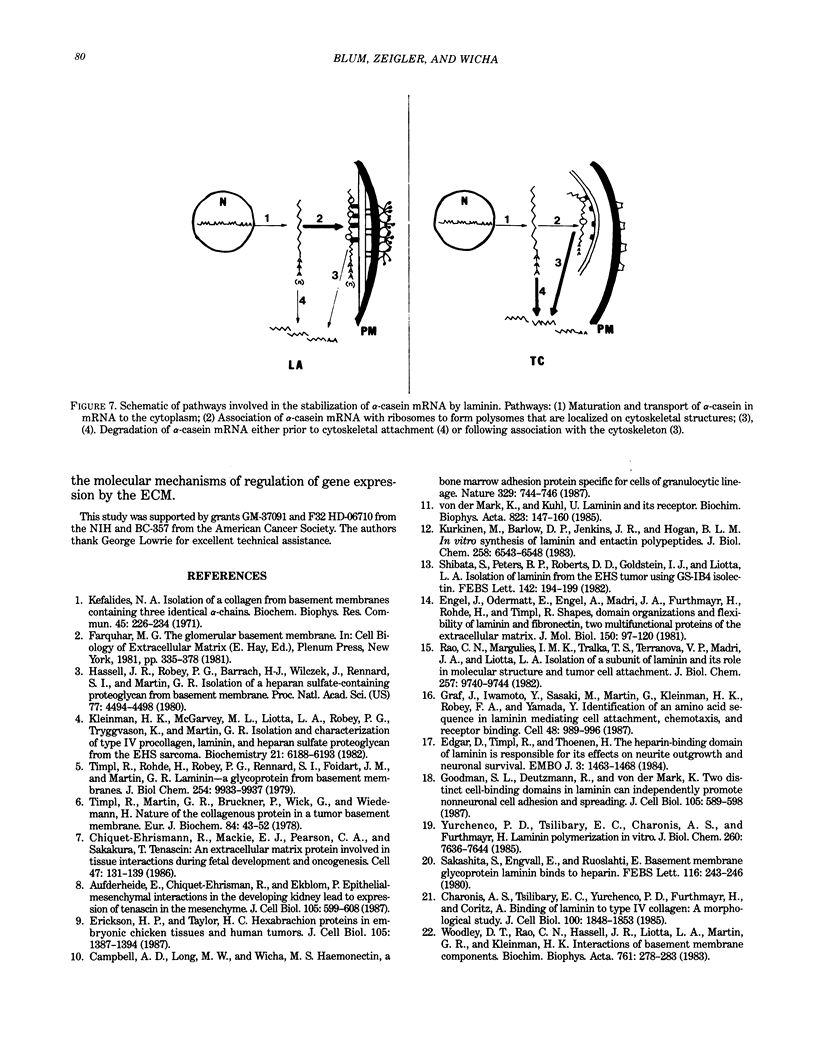
Abstract
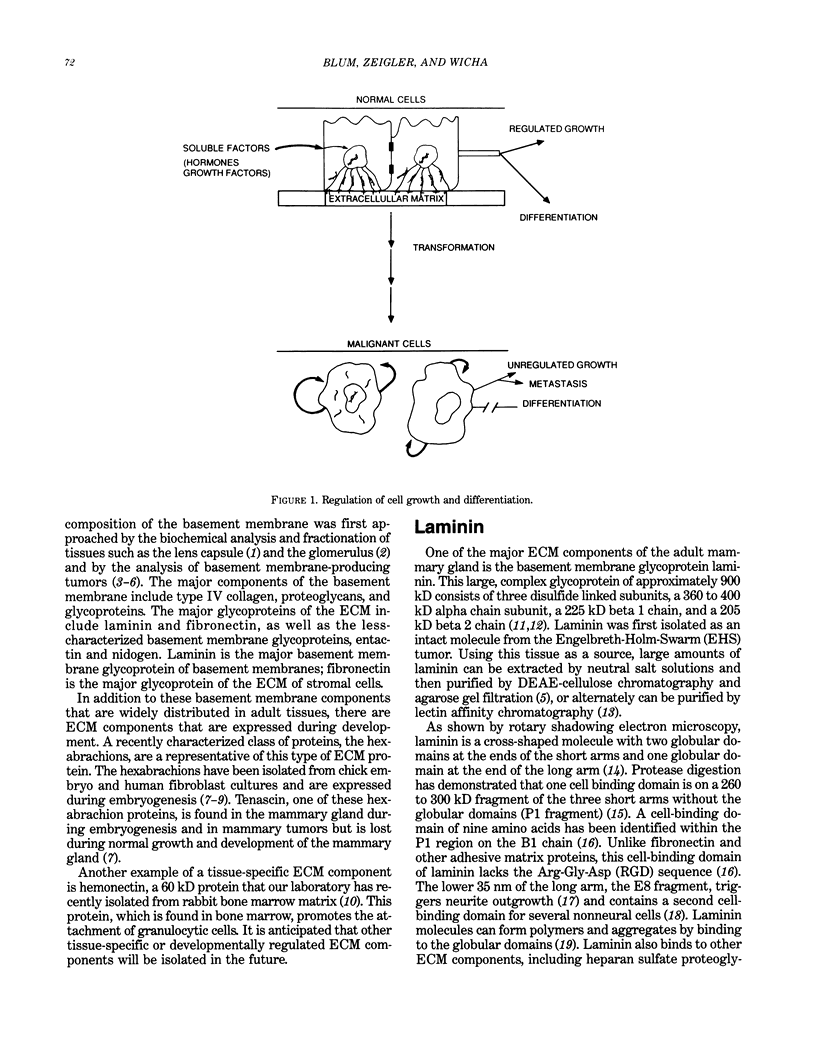
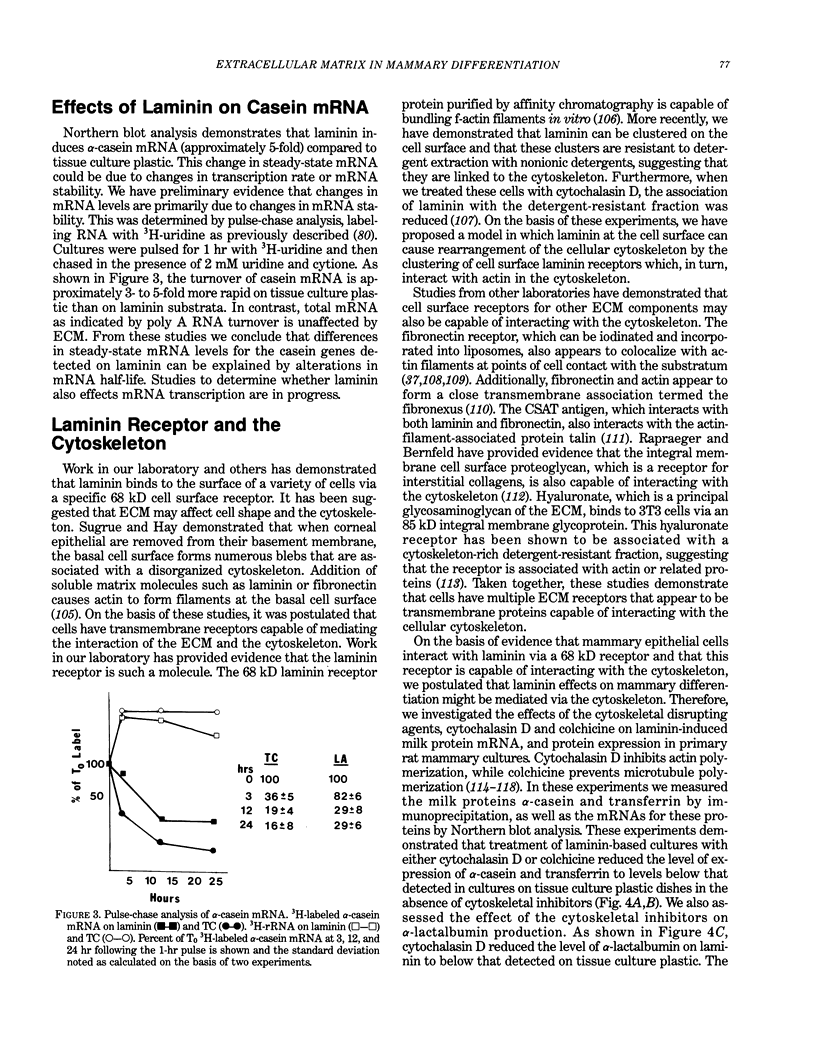
In multicellular organisms cell growth and differentiation are influenced by soluble factors, cell-cell interactions and cell-extracellular matrix interactions. We have used the rat mammary gland as a model system to study the role of extracellular matrix components in the regulation of milk protein gene expression. Since mammary epithelial cells differentiate on a basement membrane in vivo, we investigated the effects of basement membrane components on the expression of the milk protein genes, alpha-casein, alpha-lactalbumin, and transferrin. We have demonstrated that a basement membrane gel, as well as its major basement membrane component, laminin, induced alpha-casein and alpha-lactalbumin expression as much as 160-fold compared to tissue culture plastic. We demonstrate that laminin affects mRNA stability as well as having an effect on protein stability and secretion. Laminin interacts with mammary epithelial cells via an 68 kD cell surface receptor which is capable of interacting with the cellular cytoskeleton. In order to provide evidence that laminin affects on mammary differentiation are mediated through this receptor via the cytoskeleton, we examined the effects of cytoskeletal disrupting agents on milk protein gene expression. We demonstrate that cytochalasin D or colchicine selectively block laminin-mediated milk protein gene expression by affecting mRNA stability. Based on these experiments, we propose a model in which laminin affects mammary gene expression through interaction with cell surface receptors which interact with the cytoskeleton resulting in stabilization of mRNAs for milk protein genes.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andreu J. M., Timasheff S. N. Tubulin-colchicine interactions and polymerization of the complex. Ann N Y Acad Sci. 1986;466:676–689. doi: 10.1111/j.1749-6632.1986.tb38451.x. [DOI] [PubMed] [Google Scholar]
- Aufderheide E., Chiquet-Ehrismann R., Ekblom P. Epithelial-mesenchymal interactions in the developing kidney lead to expression of tenascin in the mesenchyme. J Cell Biol. 1987 Jul;105(1):599–608. doi: 10.1083/jcb.105.1.599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blum J. L., Zeigler M. E., Wicha M. S. Regulation of rat mammary gene expression by extracellular matrix components. Exp Cell Res. 1987 Dec;173(2):322–340. doi: 10.1016/0014-4827(87)90274-6. [DOI] [PubMed] [Google Scholar]
- Bonneau A. M., Darveau A., Sonenberg N. Effect of viral infection on host protein synthesis and mRNA association with the cytoplasmic cytoskeletal structure. J Cell Biol. 1985 Apr;100(4):1209–1218. doi: 10.1083/jcb.100.4.1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bornstein P., Sage H. Structurally distinct collagen types. Annu Rev Biochem. 1980;49:957–1003. doi: 10.1146/annurev.bi.49.070180.004521. [DOI] [PubMed] [Google Scholar]
- Brown S. S., Malinoff H. L., Wicha M. S. Connectin: cell surface protein that binds both laminin and actin. Proc Natl Acad Sci U S A. 1983 Oct;80(19):5927–5930. doi: 10.1073/pnas.80.19.5927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown S. S., Spudich J. A. Cytochalasin inhibits the rate of elongation of actin filament fragments. J Cell Biol. 1979 Dec;83(3):657–662. doi: 10.1083/jcb.83.3.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burwen S. J., Pitelka D. R. Secretory function of lactating mouse mammary epithelial cells cultured on collagen gels. Exp Cell Res. 1980 Apr;126(2):249–262. doi: 10.1016/0014-4827(80)90263-3. [DOI] [PubMed] [Google Scholar]
- Campbell A. D., Long M. W., Wicha M. S. Haemonectin, a bone marrow adhesion protein specific for cells of granulocyte lineage. Nature. 1987 Oct 22;329(6141):744–746. doi: 10.1038/329744a0. [DOI] [PubMed] [Google Scholar]
- Carlin B., Jaffe R., Bender B., Chung A. E. Entactin, a novel basal lamina-associated sulfated glycoprotein. J Biol Chem. 1981 May 25;256(10):5209–5214. [PubMed] [Google Scholar]
- Cervera M., Dreyfuss G., Penman S. Messenger RNA is translated when associated with the cytoskeletal framework in normal and VSV-infected HeLa cells. Cell. 1981 Jan;23(1):113–120. doi: 10.1016/0092-8674(81)90276-2. [DOI] [PubMed] [Google Scholar]
- Charonis A. S., Tsilibary E. C., Yurchenco P. D., Furthmayr H. Binding of laminin to type IV collagen: a morphological study. J Cell Biol. 1985 Jun;100(6):1848–1853. doi: 10.1083/jcb.100.6.1848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W. T., Hasegawa E., Hasegawa T., Weinstock C., Yamada K. M. Development of cell surface linkage complexes in cultured fibroblasts. J Cell Biol. 1985 Apr;100(4):1103–1114. doi: 10.1083/jcb.100.4.1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiquet-Ehrismann R., Mackie E. J., Pearson C. A., Sakakura T. Tenascin: an extracellular matrix protein involved in tissue interactions during fetal development and oncogenesis. Cell. 1986 Oct 10;47(1):131–139. doi: 10.1016/0092-8674(86)90374-0. [DOI] [PubMed] [Google Scholar]
- Chomczynski P., Qasba P., Topper Y. J. Essential role of insulin in transcription of the rat 25,000 molecular weight casein gene. Science. 1984 Dec 14;226(4680):1326–1328. doi: 10.1126/science.6390680. [DOI] [PubMed] [Google Scholar]
- Cody R. L., Wicha M. S. Clustering of cell surface laminin enhances its association with the cytoskeleton. Exp Cell Res. 1986 Jul;165(1):107–116. doi: 10.1016/0014-4827(86)90536-7. [DOI] [PubMed] [Google Scholar]
- David G., Bernfield M. R. Collagen reduces glycosaminoglycan degradation by cultured mammary epithelial cells: possible mechanism for basal lamina formation. Proc Natl Acad Sci U S A. 1979 Feb;76(2):786–790. doi: 10.1073/pnas.76.2.786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David G., Bernfield M. Type I collagen reduces the degradation of basal lamina proteoglycan by mammary epithelial cells. J Cell Biol. 1981 Oct;91(1):281–286. doi: 10.1083/jcb.91.1.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dedhar S., Ruoslahti E., Pierschbacher M. D. A cell surface receptor complex for collagen type I recognizes the Arg-Gly-Asp sequence. J Cell Biol. 1987 Mar;104(3):585–593. doi: 10.1083/jcb.104.3.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Rosso M., Cappelletti R., Viti M., Vannucchi S., Chiarugi V. Binding of the basement-membrane glycoprotein laminin to glycosaminoglycans. An affinity-chromatography study. Biochem J. 1981 Dec 1;199(3):699–704. doi: 10.1042/bj1990699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devinoy E., Houdebine L. M., Delouis C. Role of prolactin and glucocorticoids in the expression of casein genes in rabbit mammary gland organ culture. Quantification of casein mRNA. Biochim Biophys Acta. 1978 Feb 16;517(2):360–366. doi: 10.1016/0005-2787(78)90202-2. [DOI] [PubMed] [Google Scholar]
- Durkin M. E., Carlin B. E., Vergnes J., Bartos B., Merlie J., Chung A. E. Carboxyl-terminal sequence of entactin deduced from a cDNA clone. Proc Natl Acad Sci U S A. 1987 Mar;84(6):1570–1574. doi: 10.1073/pnas.84.6.1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dziadek M., Timpl R. Expression of nidogen and laminin in basement membranes during mouse embryogenesis and in teratocarcinoma cells. Dev Biol. 1985 Oct;111(2):372–382. doi: 10.1016/0012-1606(85)90491-9. [DOI] [PubMed] [Google Scholar]
- Edgar D., Timpl R., Thoenen H. The heparin-binding domain of laminin is responsible for its effects on neurite outgrowth and neuronal survival. EMBO J. 1984 Jul;3(7):1463–1468. doi: 10.1002/j.1460-2075.1984.tb01997.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emerman J. T., Burwen S. J., Pitelka D. R. Substrate properties influencing ultrastructural differentiation of mammary epithelial cells in culture. Tissue Cell. 1979;11(1):109–119. doi: 10.1016/0040-8166(79)90011-9. [DOI] [PubMed] [Google Scholar]
- Emerman J. T., Pitelka D. R. Maintenance and induction of morphological differentiation in dissociated mammary epithelium on floating collagen membranes. In Vitro. 1977 May;13(5):316–328. doi: 10.1007/BF02616178. [DOI] [PubMed] [Google Scholar]
- Engel J., Odermatt E., Engel A., Madri J. A., Furthmayr H., Rohde H., Timpl R. Shapes, domain organizations and flexibility of laminin and fibronectin, two multifunctional proteins of the extracellular matrix. J Mol Biol. 1981 Jul 25;150(1):97–120. doi: 10.1016/0022-2836(81)90326-0. [DOI] [PubMed] [Google Scholar]
- Erickson H. P., Taylor H. C. Hexabrachion proteins in embryonic chicken tissues and human tumors. J Cell Biol. 1987 Sep;105(3):1387–1394. doi: 10.1083/jcb.105.3.1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flanagan M. D., Lin S. Cytochalasins block actin filament elongation by binding to high affinity sites associated with F-actin. J Biol Chem. 1980 Feb 10;255(3):835–838. [PubMed] [Google Scholar]
- Ganguly R., Majumder P. K., Ganguly N., Banerjee M. R. The mechanism of progesterone-glucocorticoid interaction in regulation of casein gene expression. J Biol Chem. 1982 Mar 10;257(5):2182–2187. [PubMed] [Google Scholar]
- Goodman S. L., Deutzmann R., von der Mark K. Two distinct cell-binding domains in laminin can independently promote nonneuronal cell adhesion and spreading. J Cell Biol. 1987 Jul;105(1):589–598. doi: 10.1083/jcb.105.1.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graf J., Iwamoto Y., Sasaki M., Martin G. R., Kleinman H. K., Robey F. A., Yamada Y. Identification of an amino acid sequence in laminin mediating cell attachment, chemotaxis, and receptor binding. Cell. 1987 Mar 27;48(6):989–996. doi: 10.1016/0092-8674(87)90707-0. [DOI] [PubMed] [Google Scholar]
- Guyette W. A., Matusik R. J., Rosen J. M. Prolactin-mediated transcriptional and post-transcriptional control of casein gene expression. Cell. 1979 Aug;17(4):1013–1023. doi: 10.1016/0092-8674(79)90340-4. [DOI] [PubMed] [Google Scholar]
- Haeuptle M. T., Suard Y. L., Bogenmann E., Reggio H., Racine L., Kraehenbuhl J. P. Effect of cell shape change on the function and differentiation of rabbit mammary cells in culture. J Cell Biol. 1983 May;96(5):1425–1434. doi: 10.1083/jcb.96.5.1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassell J. R., Robey P. G., Barrach H. J., Wilczek J., Rennard S. I., Martin G. R. Isolation of a heparan sulfate-containing proteoglycan from basement membrane. Proc Natl Acad Sci U S A. 1980 Aug;77(8):4494–4498. doi: 10.1073/pnas.77.8.4494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobbs A. A., Richards D. A., Kessler D. J., Rosen J. M. Complex hormonal regulation of rat casein gene expression. J Biol Chem. 1982 Apr 10;257(7):3598–3605. [PubMed] [Google Scholar]
- Horwitz A., Duggan K., Buck C., Beckerle M. C., Burridge K. Interaction of plasma membrane fibronectin receptor with talin--a transmembrane linkage. Nature. 1986 Apr 10;320(6062):531–533. doi: 10.1038/320531a0. [DOI] [PubMed] [Google Scholar]
- Horwitz A., Duggan K., Greggs R., Decker C., Buck C. The cell substrate attachment (CSAT) antigen has properties of a receptor for laminin and fibronectin. J Cell Biol. 1985 Dec;101(6):2134–2144. doi: 10.1083/jcb.101.6.2134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huard T. K., Malinoff H. L., Wicha M. S. Macrophages express a plasma membrane receptor for basement membrane laminin. Am J Pathol. 1986 May;123(2):365–370. [PMC free article] [PubMed] [Google Scholar]
- Hynes R. O. Integrins: a family of cell surface receptors. Cell. 1987 Feb 27;48(4):549–554. doi: 10.1016/0092-8674(87)90233-9. [DOI] [PubMed] [Google Scholar]
- Hynes R. O., Yamada K. M. Fibronectins: multifunctional modular glycoproteins. J Cell Biol. 1982 Nov;95(2 Pt 1):369–377. doi: 10.1083/jcb.95.2.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hök M., Kjellén L., Johansson S. Cell-surface glycosaminoglycans. Annu Rev Biochem. 1984;53:847–869. doi: 10.1146/annurev.bi.53.070184.004215. [DOI] [PubMed] [Google Scholar]
- Jones W. K., Yu-Lee L. Y., Clift S. M., Brown T. L., Rosen J. M. The rat casein multigene family. Fine structure and evolution of the beta-casein gene. J Biol Chem. 1985 Jun 10;260(11):7042–7050. [PubMed] [Google Scholar]
- Kaetzel C. S., Ray D. B. Immunochemical characterization with monoclonal antibodies of three major caseins and alpha-lactalbumin from rat milk. J Dairy Sci. 1984 Jan;67(1):64–75. doi: 10.3168/jds.S0022-0302(84)81267-9. [DOI] [PubMed] [Google Scholar]
- Kefalides N. A., Alper R., Clark C. C. Biochemistry and metabolism of basement membranes. Int Rev Cytol. 1979;61:167–228. doi: 10.1016/s0074-7696(08)61998-1. [DOI] [PubMed] [Google Scholar]
- Kefalides N. A. Isolation of a collagen from basement membranes containing three identical - chains. Biochem Biophys Res Commun. 1971 Oct 1;45(1):226–234. doi: 10.1016/0006-291x(71)90073-8. [DOI] [PubMed] [Google Scholar]
- Kleinman H. K., Klebe R. J., Martin G. R. Role of collagenous matrices in the adhesion and growth of cells. J Cell Biol. 1981 Mar;88(3):473–485. doi: 10.1083/jcb.88.3.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinman H. K., McGarvey M. L., Hassell J. R., Star V. L., Cannon F. B., Laurie G. W., Martin G. R. Basement membrane complexes with biological activity. Biochemistry. 1986 Jan 28;25(2):312–318. doi: 10.1021/bi00350a005. [DOI] [PubMed] [Google Scholar]
- Kleinman H. K., McGarvey M. L., Liotta L. A., Robey P. G., Tryggvason K., Martin G. R. Isolation and characterization of type IV procollagen, laminin, and heparan sulfate proteoglycan from the EHS sarcoma. Biochemistry. 1982 Nov 23;21(24):6188–6193. doi: 10.1021/bi00267a025. [DOI] [PubMed] [Google Scholar]
- Koda J. E., Rapraeger A., Bernfield M. Heparan sulfate proteoglycans from mouse mammary epithelial cells. Cell surface proteoglycan as a receptor for interstitial collagens. J Biol Chem. 1985 Jul 5;260(13):8157–8162. [PubMed] [Google Scholar]
- Kurkinen M., Barlow D. P., Jenkins J. R., Hogan B. L. In vitro synthesis of laminin and entactin polypeptides. J Biol Chem. 1983 May 25;258(10):6543–6548. [PubMed] [Google Scholar]
- Kurkinen M., Taylor A., Garrels J. I., Hogan B. L. Cell surface-associated proteins which bind native type IV collagen or gelatin. J Biol Chem. 1984 May 10;259(9):5915–5922. [PubMed] [Google Scholar]
- Lacy B. E., Underhill C. B. The hyaluronate receptor is associated with actin filaments. J Cell Biol. 1987 Sep;105(3):1395–1404. doi: 10.1083/jcb.105.3.1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee E. Y., Lee W. H., Kaetzel C. S., Parry G., Bissell M. J. Interaction of mouse mammary epithelial cells with collagen substrata: regulation of casein gene expression and secretion. Proc Natl Acad Sci U S A. 1985 Mar;82(5):1419–1423. doi: 10.1073/pnas.82.5.1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee E. Y., Parry G., Bissell M. J. Modulation of secreted proteins of mouse mammary epithelial cells by the collagenous substrata. J Cell Biol. 1984 Jan;98(1):146–155. doi: 10.1083/jcb.98.1.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenk R., Ransom L., Kaufmann Y., Penman S. A cytoskeletal structure with associated polyribosomes obtained from HeLa cells. Cell. 1977 Jan;10(1):67–78. doi: 10.1016/0092-8674(77)90141-6. [DOI] [PubMed] [Google Scholar]
- Lesot H., Kühl U., Mark K. Isolation of a laminin-binding protein from muscle cell membranes. EMBO J. 1983;2(6):861–865. doi: 10.1002/j.1460-2075.1983.tb01514.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M. L., Aggeler J., Farson D. A., Hatier C., Hassell J., Bissell M. J. Influence of a reconstituted basement membrane and its components on casein gene expression and secretion in mouse mammary epithelial cells. Proc Natl Acad Sci U S A. 1987 Jan;84(1):136–140. doi: 10.1073/pnas.84.1.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liotta L. A., Wicha M. S., Foidart J. M., Rennard S. I., Garbisa S., Kidwell W. R. Hormonal requirements for basement membrane collagen deposition by cultured rat mammary epithelium. Lab Invest. 1979 Dec;41(6):511–518. [PubMed] [Google Scholar]
- MacLean-Fletcher S., Pollard T. D. Mechanism of action of cytochalasin B on actin. Cell. 1980 Jun;20(2):329–341. doi: 10.1016/0092-8674(80)90619-4. [DOI] [PubMed] [Google Scholar]
- Malinoff H. L., Wicha M. S. Isolation of a cell surface receptor protein for laminin from murine fibrosarcoma cells. J Cell Biol. 1983 May;96(5):1475–1479. doi: 10.1083/jcb.96.5.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Hernandez A., Fink L. M., Pierce G. B. Removal of basement membrane in the involuting breast. Lab Invest. 1976 May;34(5):455–462. [PubMed] [Google Scholar]
- Martinez-Hernandez A., Gay S., Miller E. J. Ultrastructural localization of type V collagen in rat kidney. J Cell Biol. 1982 Feb;92(2):343–349. doi: 10.1083/jcb.92.2.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta N. M., Ganguly N., Ganguly R., Banerjee M. R. Hormonal modulation of the casein gene expression in a mammogenesis-lactogenesis culture model of the whole mammary gland of the mouse. J Biol Chem. 1980 May 25;255(10):4430–4434. [PubMed] [Google Scholar]
- Modesti A., Kalebic T., Scarpa S., Togo S., Grotendorst G., Liotta L. A., Triche T. J. Type V collagen in human amnion is a 12 nm fibrillar component of the pericellular interstitium. Eur J Cell Biol. 1984 Nov;35(2):246–255. [PubMed] [Google Scholar]
- Ormerod E. J., Warburton M. J., Hughes C., Rudland P. S. Synthesis of basement membrane proteins by rat mammary epithelial cells. Dev Biol. 1983 Mar;96(1):269–275. doi: 10.1016/0012-1606(83)90328-7. [DOI] [PubMed] [Google Scholar]
- Ornelles D. A., Fey E. G., Penman S. Cytochalasin releases mRNA from the cytoskeletal framework and inhibits protein synthesis. Mol Cell Biol. 1986 May;6(5):1650–1662. doi: 10.1128/mcb.6.5.1650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parry G., Cullen B., Kaetzel C. S., Kramer R., Moss L. Regulation of differentiation and polarized secretion in mammary epithelial cells maintained in culture: extracellular matrix and membrane polarity influences. J Cell Biol. 1987 Nov;105(5):2043–2051. doi: 10.1083/jcb.105.5.2043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parry G., Lee E. Y., Farson D., Koval M., Bissell M. J. Collagenous substrata regulate the nature and distribution of glycosaminoglycans produced by differentiated cultures of mouse mammary epithelial cells. Exp Cell Res. 1985 Feb;156(2):487–499. doi: 10.1016/0014-4827(85)90556-7. [DOI] [PubMed] [Google Scholar]
- Pierschbacher M. D., Ruoslahti E. Cell attachment activity of fibronectin can be duplicated by small synthetic fragments of the molecule. Nature. 1984 May 3;309(5963):30–33. doi: 10.1038/309030a0. [DOI] [PubMed] [Google Scholar]
- Pierschbacher M., Hayman E. G., Ruoslahti E. Synthetic peptide with cell attachment activity of fibronectin. Proc Natl Acad Sci U S A. 1983 Mar;80(5):1224–1227. doi: 10.1073/pnas.80.5.1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plow E. F., Pierschbacher M. D., Ruoslahti E., Marguerie G. A., Ginsberg M. H. The effect of Arg-Gly-Asp-containing peptides on fibrinogen and von Willebrand factor binding to platelets. Proc Natl Acad Sci U S A. 1985 Dec;82(23):8057–8061. doi: 10.1073/pnas.82.23.8057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pytela R., Pierschbacher M. D., Ginsberg M. H., Plow E. F., Ruoslahti E. Platelet membrane glycoprotein IIb/IIIa: member of a family of Arg-Gly-Asp--specific adhesion receptors. Science. 1986 Mar 28;231(4745):1559–1562. doi: 10.1126/science.2420006. [DOI] [PubMed] [Google Scholar]
- Pytela R., Pierschbacher M. D., Ruoslahti E. A 125/115-kDa cell surface receptor specific for vitronectin interacts with the arginine-glycine-aspartic acid adhesion sequence derived from fibronectin. Proc Natl Acad Sci U S A. 1985 Sep;82(17):5766–5770. doi: 10.1073/pnas.82.17.5766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pytela R., Pierschbacher M. D., Ruoslahti E. Identification and isolation of a 140 kd cell surface glycoprotein with properties expected of a fibronectin receptor. Cell. 1985 Jan;40(1):191–198. doi: 10.1016/0092-8674(85)90322-8. [DOI] [PubMed] [Google Scholar]
- Rao C. N., Margulies I. M., Tralka T. S., Terranova V. P., Madri J. A., Liotta L. A. Isolation of a subunit of laminin and its role in molecular structure and tumor cell attachment. J Biol Chem. 1982 Aug 25;257(16):9740–9744. [PubMed] [Google Scholar]
- Rao N. C., Barsky S. H., Terranova V. P., Liotta L. A. Isolation of a tumor cell laminin receptor. Biochem Biophys Res Commun. 1983 Mar 29;111(3):804–808. doi: 10.1016/0006-291x(83)91370-0. [DOI] [PubMed] [Google Scholar]
- Rapraeger A., Jalkanen M., Bernfield M. Cell surface proteoglycan associates with the cytoskeleton at the basolateral cell surface of mouse mammary epithelial cells. J Cell Biol. 1986 Dec;103(6 Pt 2):2683–2696. doi: 10.1083/jcb.103.6.2683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards D. A., Blackburn D. E., Rosen J. M. Restriction enzyme mapping and heteroduplex analysis of the rat milk protein cDNA clones. J Biol Chem. 1981 Jan 10;256(1):533–538. [PubMed] [Google Scholar]
- Richards D. A., Rodgers J. R., Supowit S. C., Rosen J. M. Construction and preliminary characterization of the rat casein and alpha-lactalbumin cDNA clones. J Biol Chem. 1981 Jan 10;256(1):526–532. [PubMed] [Google Scholar]
- Roberts D. D., Rao C. N., Magnani J. L., Spitalnik S. L., Liotta L. A., Ginsburg V. Laminin binds specifically to sulfated glycolipids. Proc Natl Acad Sci U S A. 1985 Mar;82(5):1306–1310. doi: 10.1073/pnas.82.5.1306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocha V., Ringo D. L., Read D. B. Casein production during differentiation of mammary epithelial cells in collagen gel culture. Exp Cell Res. 1985 Jul;159(1):201–210. doi: 10.1016/s0014-4827(85)80049-5. [DOI] [PubMed] [Google Scholar]
- Rosen J. M., Matusik R. J., Richards D. A., Gupta P., Rodgers J. R. Multihormonal regulation of casein gene expression at the transcriptional and posttransciptional levels in the mammary gland. Recent Prog Horm Res. 1980;36:157–193. doi: 10.1016/b978-0-12-571136-4.50011-5. [DOI] [PubMed] [Google Scholar]
- Ruoslahti E., Engvall E., Hayman E. G. Fibronectin: current concepts of its structure and functions. Coll Relat Res. 1981;1(1):95–128. doi: 10.1016/s0174-173x(80)80011-2. [DOI] [PubMed] [Google Scholar]
- Ruoslahti E., Pierschbacher M. D. Arg-Gly-Asp: a versatile cell recognition signal. Cell. 1986 Feb 28;44(4):517–518. doi: 10.1016/0092-8674(86)90259-x. [DOI] [PubMed] [Google Scholar]
- Sakashita S., Engvall E., Ruoslahti E. Basement membrane glycoprotein laminin binds to heparin. FEBS Lett. 1980 Jul 28;116(2):243–246. doi: 10.1016/0014-5793(80)80654-5. [DOI] [PubMed] [Google Scholar]
- Salomon D. S., Liotta L. A., Kidwell W. R. Differential response to growth factor by rat mammary epithelium plated on different collagen substrata in serum-free medium. Proc Natl Acad Sci U S A. 1981 Jan;78(1):382–386. doi: 10.1073/pnas.78.1.382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schliwa M. Action of cytochalasin D on cytoskeletal networks. J Cell Biol. 1982 Jan;92(1):79–91. doi: 10.1083/jcb.92.1.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shannon J. M., Pitelka D. R. The influence of cell shape on the induction of functional differentiation in mouse mammary cells in vitro. In Vitro. 1981 Nov;17(11):1016–1028. doi: 10.1007/BF02618428. [DOI] [PubMed] [Google Scholar]
- Singer I. I., Kawka D. W., Scott S., Mumford R. A., Lark M. W. The fibronectin cell attachment sequence Arg-Gly-Asp-Ser promotes focal contact formation during early fibroblast attachment and spreading. J Cell Biol. 1987 Mar;104(3):573–584. doi: 10.1083/jcb.104.3.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer I. I. The fibronexus: a transmembrane association of fibronectin-containing fibers and bundles of 5 nm microfilaments in hamster and human fibroblasts. Cell. 1979 Mar;16(3):675–685. doi: 10.1016/0092-8674(79)90040-0. [DOI] [PubMed] [Google Scholar]
- Sporn M. B., Roberts A. B., Wakefield L. M., de Crombrugghe B. Some recent advances in the chemistry and biology of transforming growth factor-beta. J Cell Biol. 1987 Sep;105(3):1039–1045. doi: 10.1083/jcb.105.3.1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamatoglou S. C., Keller J. M. Interactions of cellular glycosaminoglycans with plasma fibronectin and collagen. Biochim Biophys Acta. 1982 Oct 28;719(1):90–97. doi: 10.1016/0304-4165(82)90311-7. [DOI] [PubMed] [Google Scholar]
- Suard Y. M., Haeuptle M. T., Farinon E., Kraehenbuhl J. P. Cell proliferation and milk protein gene expression in rabbit mammary cell cultures. J Cell Biol. 1983 May;96(5):1435–1442. doi: 10.1083/jcb.96.5.1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugrue S. P., Hay E. D. Response of basal epithelial cell surface and Cytoskeleton to solubilized extracellular matrix molecules. J Cell Biol. 1981 Oct;91(1):45–54. doi: 10.1083/jcb.91.1.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terranova V. P., Rao C. N., Kalebic T., Margulies I. M., Liotta L. A. Laminin receptor on human breast carcinoma cells. Proc Natl Acad Sci U S A. 1983 Jan;80(2):444–448. doi: 10.1073/pnas.80.2.444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teyssot B., Houdebine L. M. Role of progesterone and glucocorticoids in the transcription of the beta-casein and 28-S ribosomal genes in the rabbit mammary gland. Eur J Biochem. 1981 Mar;114(3):597–608. doi: 10.1111/j.1432-1033.1981.tb05186.x. [DOI] [PubMed] [Google Scholar]
- Timpl R., Dziadek M., Fujiwara S., Nowack H., Wick G. Nidogen: a new, self-aggregating basement membrane protein. Eur J Biochem. 1983 Dec 15;137(3):455–465. doi: 10.1111/j.1432-1033.1983.tb07849.x. [DOI] [PubMed] [Google Scholar]
- Timpl R., Martin G. R., Bruckner P., Wick G., Wiedemann H. Nature of the collagenous protein in a tumor basement membrane. Eur J Biochem. 1978 Mar;84(1):43–52. doi: 10.1111/j.1432-1033.1978.tb12139.x. [DOI] [PubMed] [Google Scholar]
- Timpl R., Rohde H., Robey P. G., Rennard S. I., Foidart J. M., Martin G. R. Laminin--a glycoprotein from basement membranes. J Biol Chem. 1979 Oct 10;254(19):9933–9937. [PubMed] [Google Scholar]
- Timpl R., Wiedemann H., van Delden V., Furthmayr H., Kühn K. A network model for the organization of type IV collagen molecules in basement membranes. Eur J Biochem. 1981 Nov;120(2):203–211. doi: 10.1111/j.1432-1033.1981.tb05690.x. [DOI] [PubMed] [Google Scholar]
- Tryggvason K., Robey P. G., Martin G. R. Biosynthesis of type IV procollagens. Biochemistry. 1980 Apr 1;19(7):1284–1289. doi: 10.1021/bi00548a003. [DOI] [PubMed] [Google Scholar]
- Warburton M. J., Mitchell D., Ormerod E. J., Rudland P. Distribution of myoepithelial cells and basement membrane proteins in the resting, pregnant, lactating, and involuting rat mammary gland. J Histochem Cytochem. 1982 Jul;30(7):667–676. doi: 10.1177/30.7.6179984. [DOI] [PubMed] [Google Scholar]
- Warburton M. J., Monaghan P., Ferns S. A., Hughes C. M., Rudland P. S. Distribution and synthesis of type V collagen in the rat mammary gland. J Histochem Cytochem. 1983 Nov;31(11):1265–1273. doi: 10.1177/31.11.6352797. [DOI] [PubMed] [Google Scholar]
- Warburton M. J., Monaghan P., Ferns S. A., Rudland P. S., Perusinghe N., Chung A. E. Distribution of entactin in the basement membrane of the rat mammary gland. Evidence for a non-epithelial origin. Exp Cell Res. 1984 May;152(1):240–254. doi: 10.1016/0014-4827(84)90249-0. [DOI] [PubMed] [Google Scholar]
- Wewer U. M., Liotta L. A., Jaye M., Ricca G. A., Drohan W. N., Claysmith A. P., Rao C. N., Wirth P., Coligan J. E., Albrechtsen R. Altered levels of laminin receptor mRNA in various human carcinoma cells that have different abilities to bind laminin. Proc Natl Acad Sci U S A. 1986 Oct;83(19):7137–7141. doi: 10.1073/pnas.83.19.7137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wicha M. S., Liotta L. A., Garbisa S., Kidwell W. R. Basement membrane collagen requirements for attachment and growth of mammary epithelium. Exp Cell Res. 1979 Nov;124(1):181–190. doi: 10.1016/0014-4827(79)90268-4. [DOI] [PubMed] [Google Scholar]
- Wicha M. S., Liotta L. A., Vonderhaar B. K., Kidwell W. R. Effects of inhibition of basement membrane collagen deposition on rat mammary gland development. Dev Biol. 1980 Dec;80(2):253–256. doi: 10.1016/0012-1606(80)90402-9. [DOI] [PubMed] [Google Scholar]
- Wicha M. S., Lowrie G., Kohn E., Bagavandoss P., Mahn T. Extracellular matrix promotes mammary epithelial growth and differentiation in vitro. Proc Natl Acad Sci U S A. 1982 May;79(10):3213–3217. doi: 10.1073/pnas.79.10.3213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodley D. T., Rao C. N., Hassell J. R., Liotta L. A., Martin G. R., Kleinman H. K. Interactions of basement membrane components. Biochim Biophys Acta. 1983 Dec 27;761(3):278–283. doi: 10.1016/0304-4165(83)90077-6. [DOI] [PubMed] [Google Scholar]
- Yoon P. S., Boxer L. A., Mayo L. A., Yang A. Y., Wicha M. S. Human neutrophil laminin receptors: activation-dependent receptor expression. J Immunol. 1987 Jan 1;138(1):259–265. [PubMed] [Google Scholar]
- Yu-Lee L. Y., Rosen J. M. The rat casein multigene family. I. Fine structure of the gamma-casein gene. J Biol Chem. 1983 Sep 10;258(17):10794–10804. [PubMed] [Google Scholar]
- Yurchenco P. D., Tsilibary E. C., Charonis A. S., Furthmayr H. Laminin polymerization in vitro. Evidence for a two-step assembly with domain specificity. J Biol Chem. 1985 Jun 25;260(12):7636–7644. [PubMed] [Google Scholar]
- von der Mark K., Kühl U. Laminin and its receptor. Biochim Biophys Acta. 1985 Dec 17;823(2):147–160. doi: 10.1016/0304-419x(85)90010-1. [DOI] [PubMed] [Google Scholar]