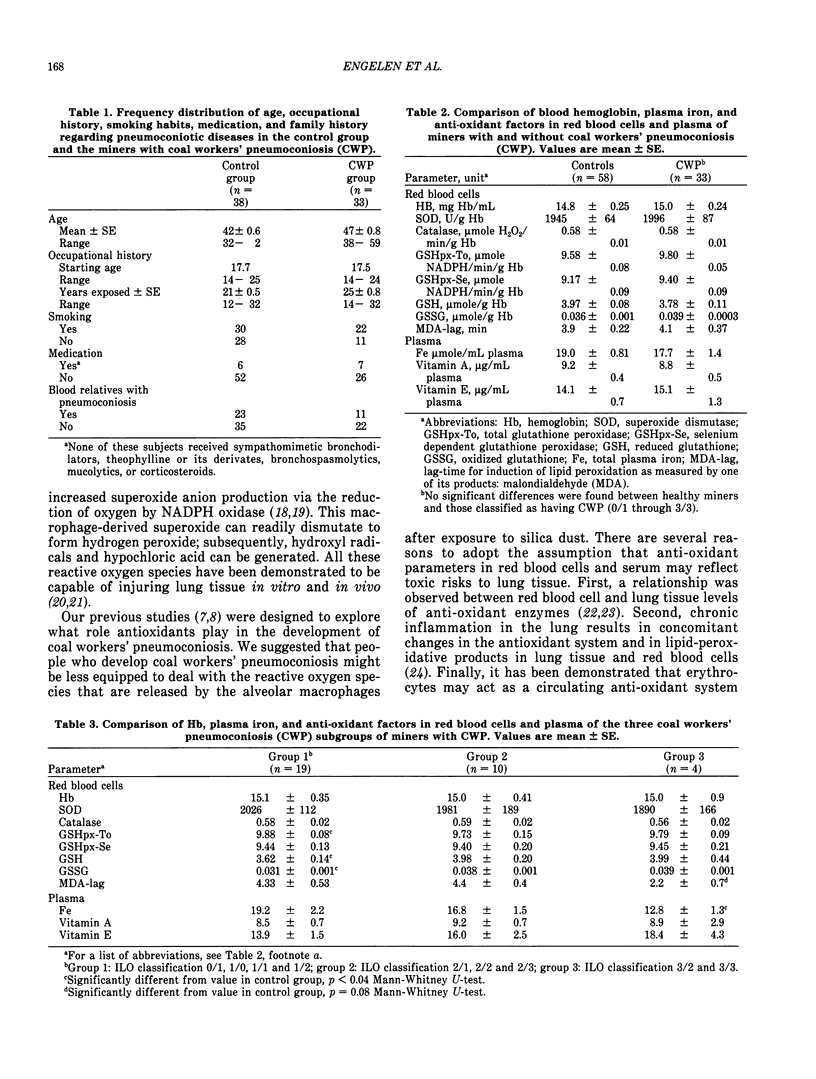
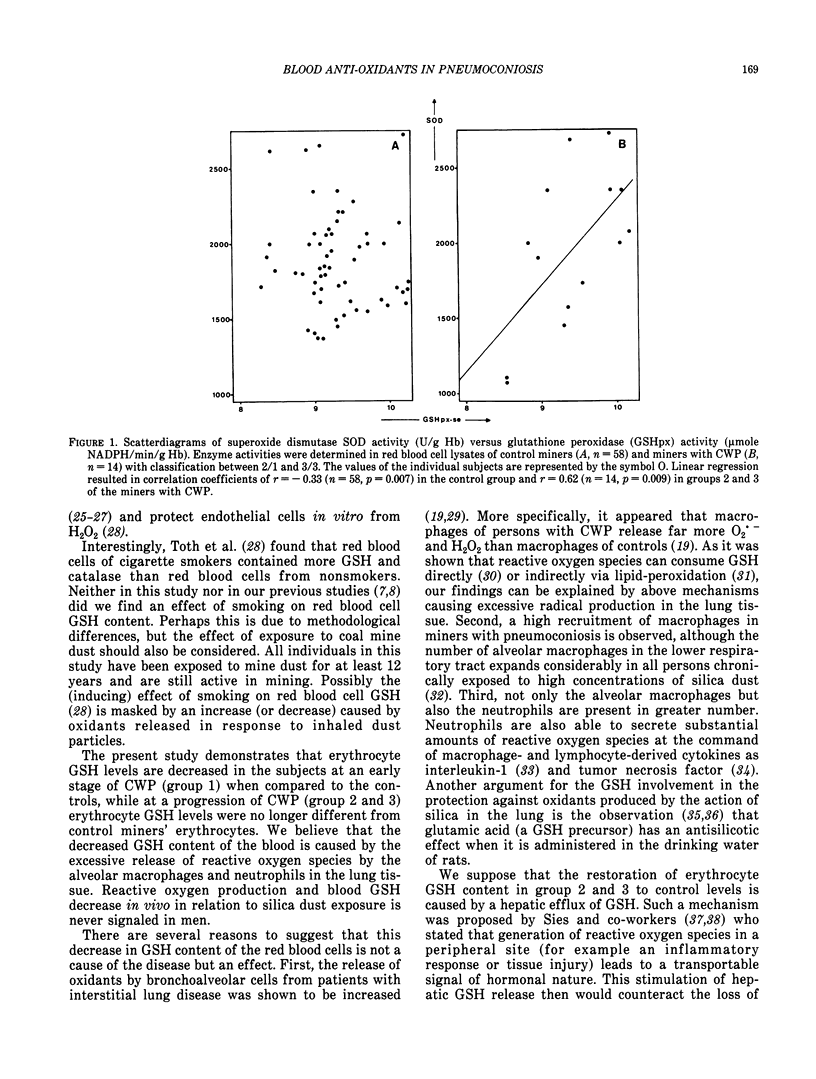
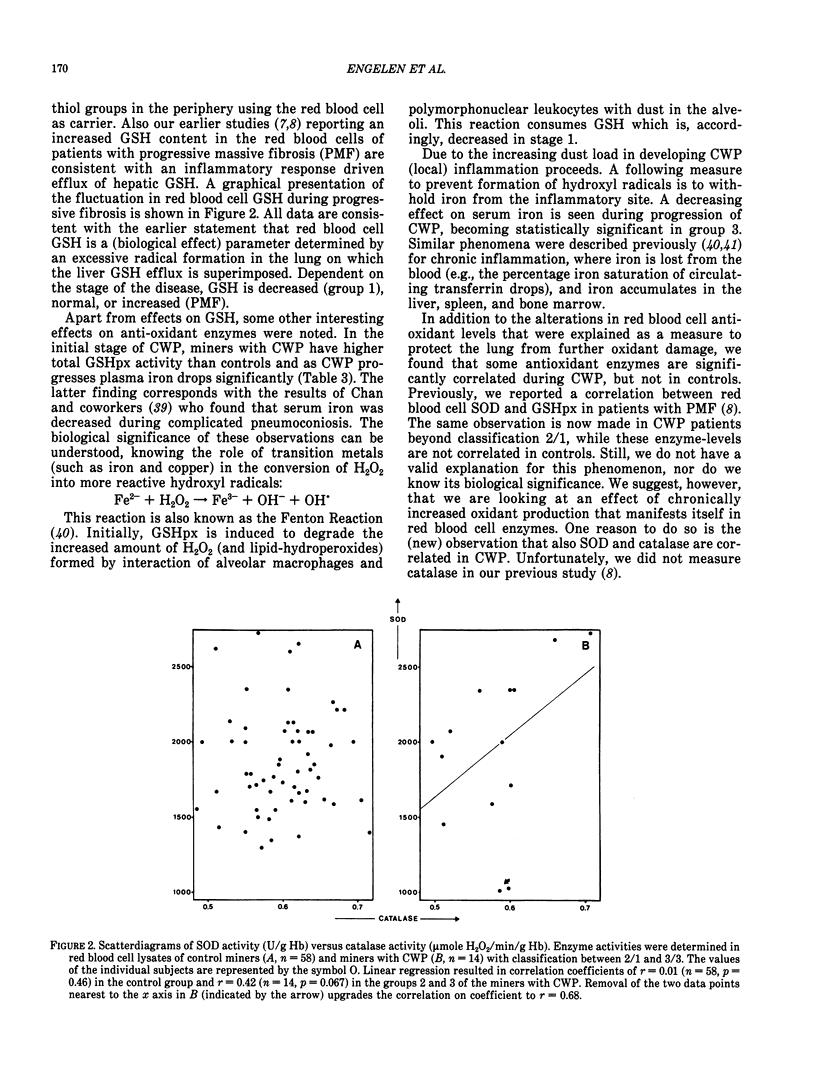
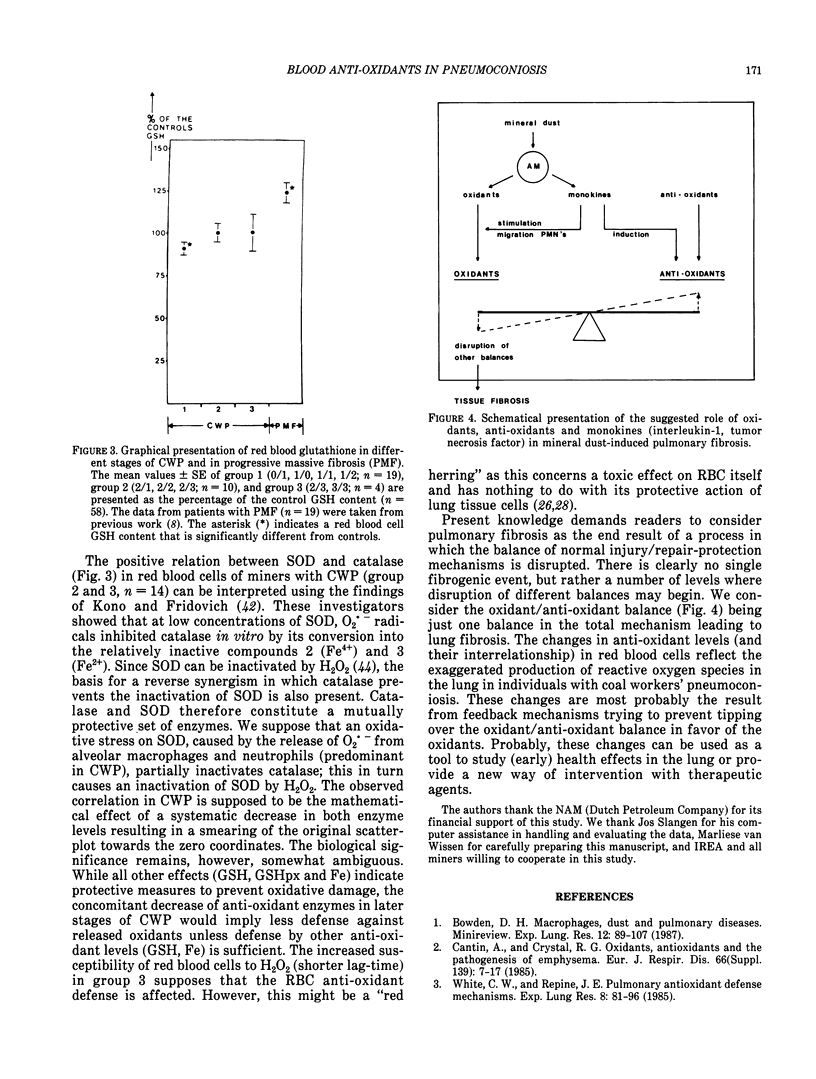
Abstract
The pneumoconioses are associated with chronic inflammatory processes during which increased amounts of reactive oxygen species are formed in the lower respiratory tract. To characterize the effect(s) of these processes on the defense system against free radicals, we studied 91 individuals with long-term occupational exposure to coal mine dust. Thirty-one subjects were classified with radiological evidence to be pneumoconiotics, while 58 control miners had no pulmonary disorders. We measured antioxidant parameters in red blood cells, considering the latter to reflect the oxidative stress in the lung. Glutathione levels were significantly decreased (p = 0.04) in red blood cells of miners with coal workers' pneumoconiosis with radiograph classification 0/1 to 2/1, while in miners with classification 3/2 to 3/3, the plasma iron concentrations were significantly decreased (p = 0.04). Moreover, some factors of the anti-oxidant system (superoxide dismutase, catalase, glutathione peroxidase) were correlated in the diseased but not in the control miners. Taken together, all data support the role of the erythrocyte as a circulating anti-oxidant carrier and also that changes in red blood cell anti-oxidant factors reflect the oxidative stress imposed by the pneumoconiotic (inflammatory) processes in the lung.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aebi H. Catalase in vitro. Methods Enzymol. 1984;105:121–126. doi: 10.1016/s0076-6879(84)05016-3. [DOI] [PubMed] [Google Scholar]
- Agar N. S., Sadrzadeh S. M., Hallaway P. E., Eaton J. W. Erythrocyte catalase. A somatic oxidant defense? J Clin Invest. 1986 Jan;77(1):319–321. doi: 10.1172/JCI112294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borm P. J., Bast A., Wouters E. F., Slangen J. J., Swaen G. M., De Boorder T. J. Red blood cell anti-oxidant parameters in healthy elderly subjects versus silicosis patients. Free Radic Res Commun. 1987;3(1-5):117–127. doi: 10.3109/10715768709069777. [DOI] [PubMed] [Google Scholar]
- Borm P. J., Bast A., Wouters E. F., Slangen J. J., Swaen G. M., de Boorder T. Red blood cell anti-oxidant parameters in silicosis. Int Arch Occup Environ Health. 1986;58(3):235–244. doi: 10.1007/BF00432106. [DOI] [PubMed] [Google Scholar]
- Bowden D. H. Macrophages, dust, and pulmonary diseases. Exp Lung Res. 1987;12(2):89–107. doi: 10.3109/01902148709062834. [DOI] [PubMed] [Google Scholar]
- Cantin A., Crystal R. G. Oxidants, antioxidants and the pathogenesis of emphysema. Eur J Respir Dis Suppl. 1985;139:7–17. [PubMed] [Google Scholar]
- Chan B. W. Serum iron and iron kinetics in coalworkers with complicated pneumoconiosis. Br J Ind Med. 1969 Jan;26(1):65–70. doi: 10.1136/oem.26.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuesta Sanz D., Castro Santa-Cruz M. Simultaneous measurement of retinol and alpha-tocopherol in human serum by high-performance liquid chromatography with ultraviolet detection. J Chromatogr. 1986 Jul 11;380(1):140–144. doi: 10.1016/s0378-4347(00)83634-8. [DOI] [PubMed] [Google Scholar]
- DiGregorio K. A., Cilento E. V., Lantz R. C. Measurement of superoxide release from single pulmonary alveolar macrophages. Am J Physiol. 1987 Jun;252(6 Pt 1):C677–C683. doi: 10.1152/ajpcell.1987.252.6.C677. [DOI] [PubMed] [Google Scholar]
- Gery I., Davies P., Derr J., Krett N., Barranger J. A. Relationship between production and release of lymphocyte-activating factor (interleukin 1) by murine macrophages. 1. Effects of various agents. Cell Immunol. 1981 Nov 1;64(2):293–303. doi: 10.1016/0008-8749(81)90481-0. [DOI] [PubMed] [Google Scholar]
- Halliwell B., Gutteridge J. M. Oxygen toxicity, oxygen radicals, transition metals and disease. Biochem J. 1984 Apr 1;219(1):1–14. doi: 10.1042/bj2190001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heppleston A. G. Pulmonary toxicology of silica, coal and asbestos. Environ Health Perspect. 1984 Apr;55:111–127. doi: 10.1289/ehp.8455111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hissin P. J., Hilf R. A fluorometric method for determination of oxidized and reduced glutathione in tissues. Anal Biochem. 1976 Jul;74(1):214–226. doi: 10.1016/0003-2697(76)90326-2. [DOI] [PubMed] [Google Scholar]
- Johnson K. J., Fantone J. C., 3rd, Kaplan J., Ward P. A. In vivo damage of rat lungs by oxygen metabolites. J Clin Invest. 1981 Apr;67(4):983–993. doi: 10.1172/JCI110149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katsnelson B. A., Polzik E. V., Privalova L. I. Some aspects of the problem of individual predisposition to silicosis. Environ Health Perspect. 1986 Sep;68:175–185. doi: 10.1289/ehp.8668175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kono Y., Fridovich I. Superoxide radical inhibits catalase. J Biol Chem. 1982 May 25;257(10):5751–5754. [PubMed] [Google Scholar]
- Martin W. J., 2nd, Gadek J. E., Hunninghake G. W., Crystal R. G. Oxidant injury of lung parenchymal cells. J Clin Invest. 1981 Nov;68(5):1277–1288. doi: 10.1172/JCI110374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCord J. M., Fridovich I. Superoxide dismutase. An enzymic function for erythrocuprein (hemocuprein). J Biol Chem. 1969 Nov 25;244(22):6049–6055. [PubMed] [Google Scholar]
- Minami M., Koshi K., Homma K., Suzuki Y. Changes of the activities of superoxide dismutase after exposure to the fume of heavy metals and the significance of zinc in the tissue. Arch Toxicol. 1982 Mar;49(3-4):215–225. doi: 10.1007/BF00347869. [DOI] [PubMed] [Google Scholar]
- Morosova K. I., Aronova G. V., Katsnelson B. A., Velichkovski B. T., Genkin A. M., Elnichnykh L. N., Privalova L. I. On the defensive action of glutamate against the cytotoxicity and fibrogenicity of quartz dust. Br J Ind Med. 1982 Aug;39(3):244–252. doi: 10.1136/oem.39.3.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morosova K. I., Katsnelson B. A., Rotenberg YuS, Belobragina G. V. A further experimental study of the antisilicotic effect of glutamate. Br J Ind Med. 1984 Nov;41(4):518–525. doi: 10.1136/oem.41.4.518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathan C. F. Neutrophil activation on biological surfaces. Massive secretion of hydrogen peroxide in response to products of macrophages and lymphocytes. J Clin Invest. 1987 Dec;80(6):1550–1560. doi: 10.1172/JCI113241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riley D. J., Kerr J. S. Oxidant injury of the extracellular matrix: potential role in the pathogenesis of pulmonary emphysema. Lung. 1985;163(1):1–13. doi: 10.1007/BF02713801. [DOI] [PubMed] [Google Scholar]
- Rom W. N., Bitterman P. B., Rennard S. I., Cantin A., Crystal R. G. Characterization of the lower respiratory tract inflammation of nonsmoking individuals with interstitial lung disease associated with chronic inhalation of inorganic dusts. Am Rev Respir Dis. 1987 Dec;136(6):1429–1434. doi: 10.1164/ajrccm/136.6.1429. [DOI] [PubMed] [Google Scholar]
- Ross D., Cotgreave I., Moldéus P. The interaction of reduced glutathione with active oxygen species generated by xanthine-oxidase-catalyzed metabolism of xanthine. Biochim Biophys Acta. 1985 Sep 6;841(3):278–282. doi: 10.1016/0304-4165(85)90069-8. [DOI] [PubMed] [Google Scholar]
- Sies H., Cadenas E. Oxidative stress: damage to intact cells and organs. Philos Trans R Soc Lond B Biol Sci. 1985 Dec 17;311(1152):617–631. doi: 10.1098/rstb.1985.0168. [DOI] [PubMed] [Google Scholar]
- Sies H., Graf P. Hepatic thiol and glutathione efflux under the influence of vasopressin, phenylephrine and adrenaline. Biochem J. 1985 Mar 1;226(2):545–549. doi: 10.1042/bj2260545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stocks J., Dormandy T. L. The autoxidation of human red cell lipids induced by hydrogen peroxide. Br J Haematol. 1971 Jan;20(1):95–111. doi: 10.1111/j.1365-2141.1971.tb00790.x. [DOI] [PubMed] [Google Scholar]
- Toth K. M., Berger E. M., Beehler C. J., Repine J. E. Erythrocytes from cigarette smokers contain more glutathione and catalase and protect endothelial cells from hydrogen peroxide better than do erythrocytes from nonsmokers. Am Rev Respir Dis. 1986 Aug;134(2):281–284. doi: 10.1164/arrd.1986.134.2.281. [DOI] [PubMed] [Google Scholar]
- White C. W., Mimmack R. F., Repine J. E. Accumulation of lung tissue oxidized glutathione (GSSG) as a marker of oxidant induced lung injury. Chest. 1986 Mar;89(3 Suppl):111S–113S. doi: 10.1378/chest.89.3_supplement.111s. [DOI] [PubMed] [Google Scholar]
- White C. W., Repine J. E. Pulmonary antioxidant defense mechanisms. Exp Lung Res. 1985;8(2-3):81–96. doi: 10.3109/01902148509057515. [DOI] [PubMed] [Google Scholar]


