Abstract
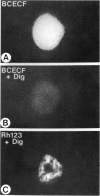
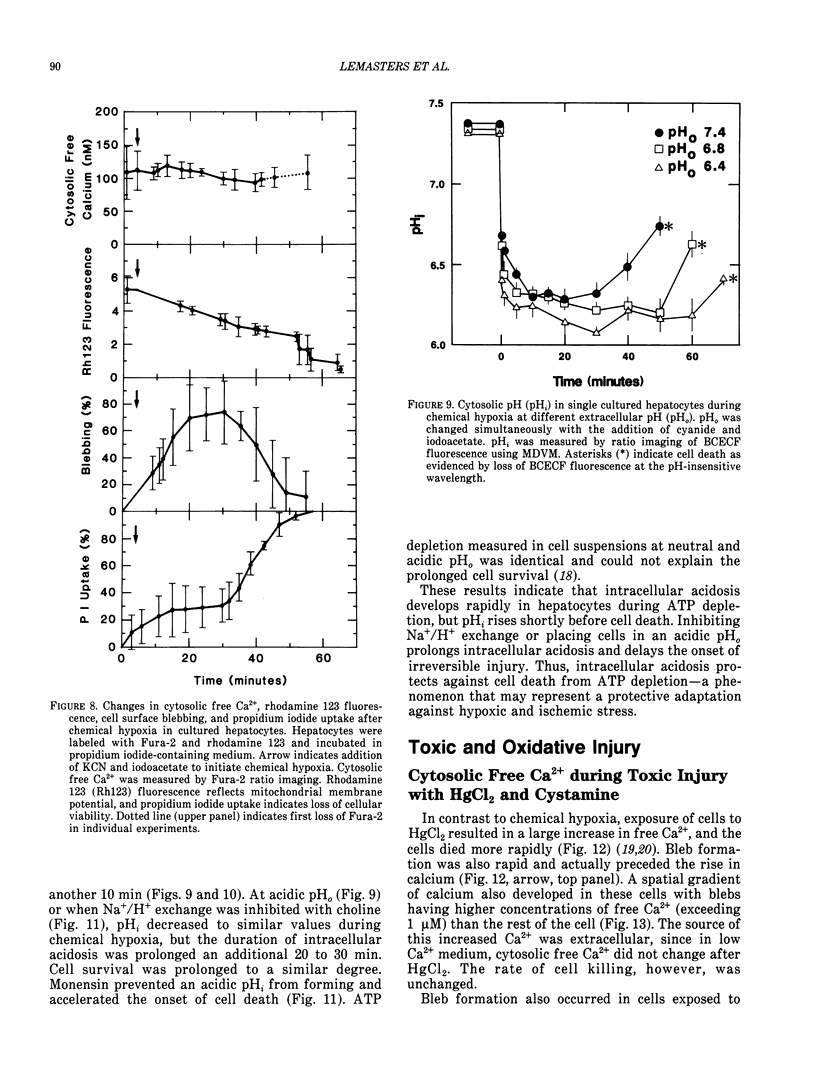
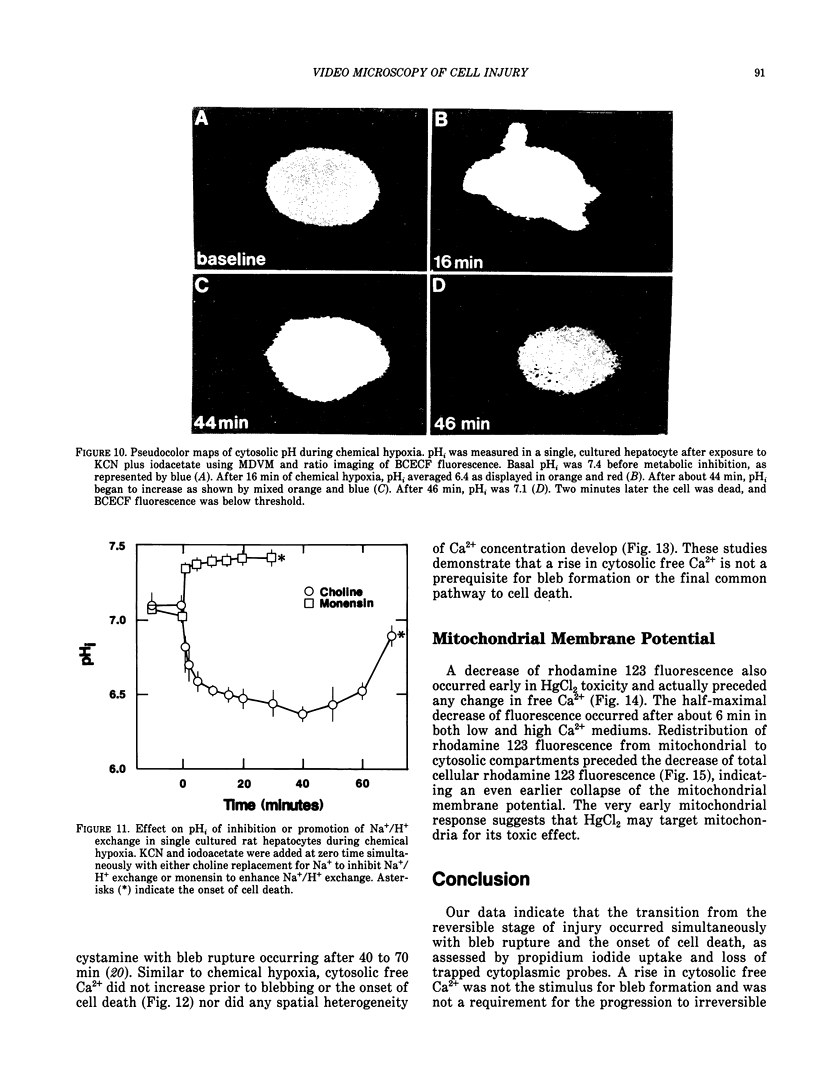
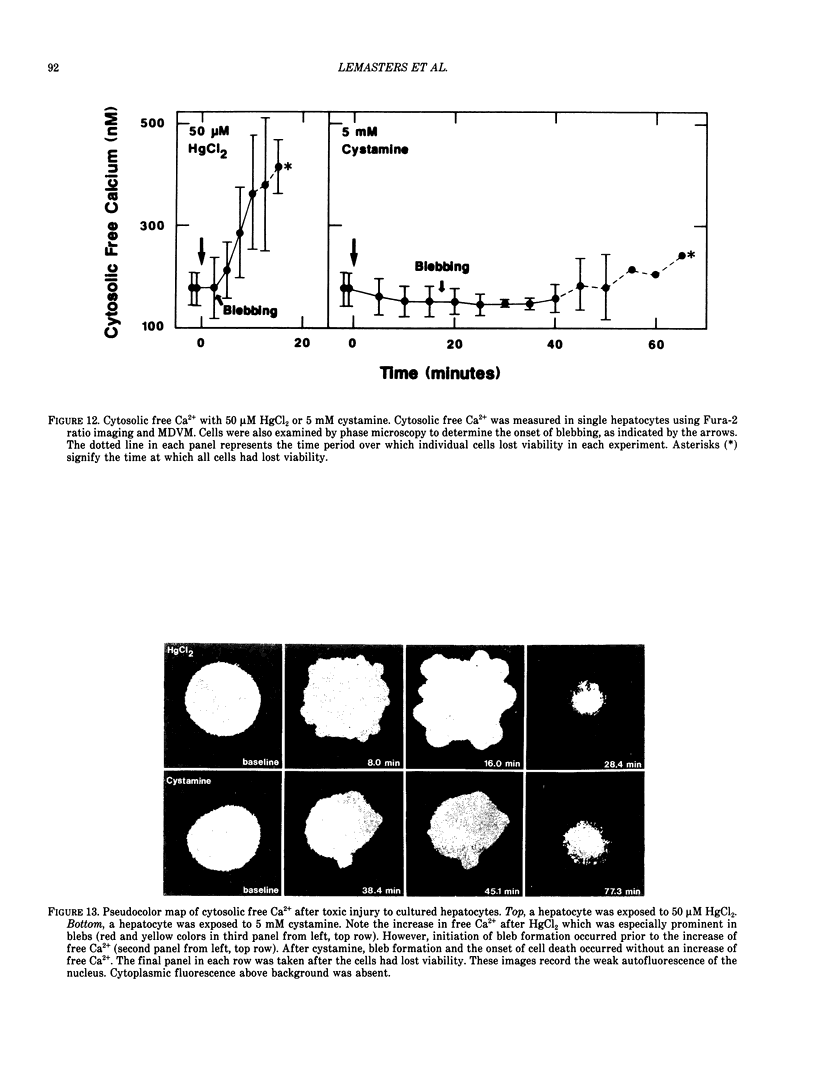
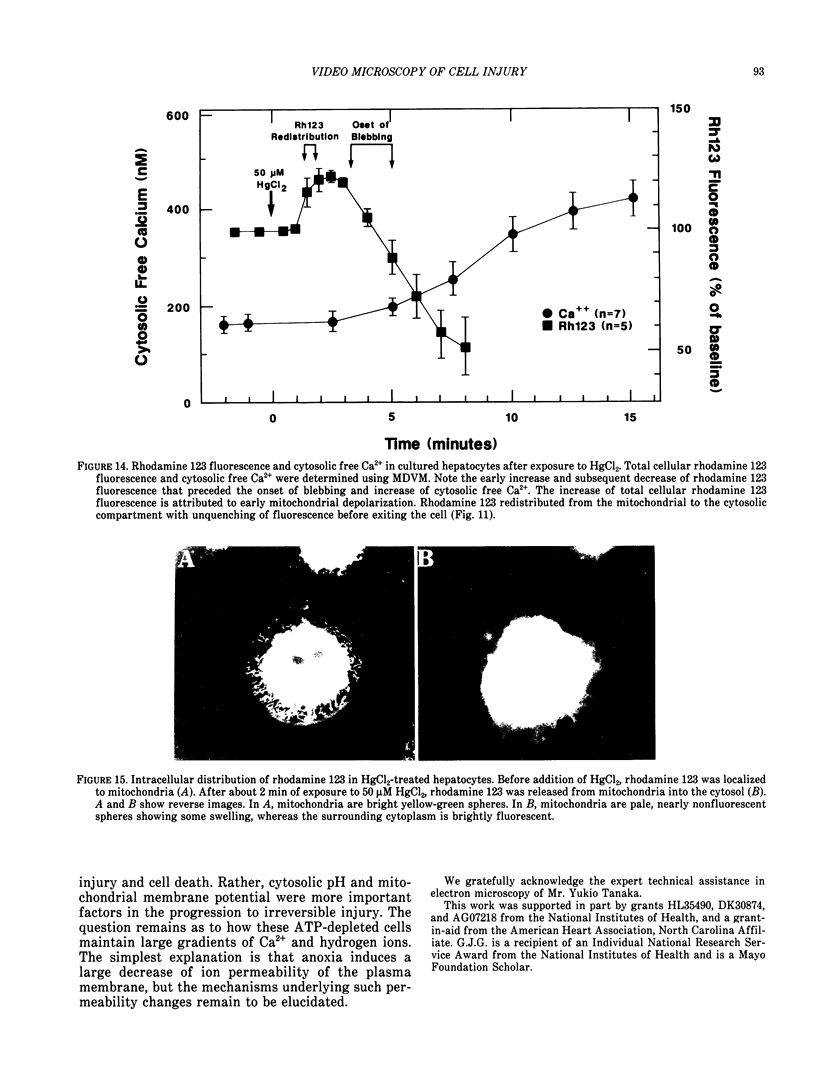
There is no clear picture of the critical events that lead to the transition from reversible to irreversible injury. Many studies have suggested that a rise in cytosolic free Ca2+ initiates plasma membrane bleb formation and a sequence of events that lead ultimately to cell death. In recent studies, we have measured changes in cytosolic free Ca2+, mitochondrial membrane potential, cytosolic pH, and cell surface blebbing in relation to the onset of irreversible injury and cell death following anoxic and toxic injury to single hepatocytes by using multiparameter digitized video microscopy (MDVM). MDVM is an emerging new technology that permits single living cells to be labeled with multiple probes whose fluorescence is responsive to specific cellular parameters of interest. Fluorescence images specific for each probe are collected over time, digitized, and stored. Image analysis and processing then permits quantitation of the spatial distribution of the various parameters with the single living cells. Our results indicate the following: The formation of plasma membrane blebs accompanies all types of injury in hepatocytes. Cell death is a rapid event initiated by rupture of a plasma membrane bleb, and it is coincident with the onset of irreversible injury. An increase of cytosolic free Ca2+ is not the stimulus for bleb formation or the final common pathway leading to cell death. A decrease of mitochondrial membrane potential precedes the loss of cell viability. Cytosolic pH falls by more than 1 pH unit during chemical hypoxia. This acidosis protects against the onset of cell death.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersson B. S., Aw T. Y., Jones D. P. Mitochondrial transmembrane potential and pH gradient during anoxia. Am J Physiol. 1987 Apr;252(4 Pt 1):C349–C355. doi: 10.1152/ajpcell.1987.252.4.C349. [DOI] [PubMed] [Google Scholar]
- Becker P. L., Fay F. S. Photobleaching of fura-2 and its effect on determination of calcium concentrations. Am J Physiol. 1987 Oct;253(4 Pt 1):C613–C618. doi: 10.1152/ajpcell.1987.253.4.C613. [DOI] [PubMed] [Google Scholar]
- Bellomo G., Orrenius S. Altered thiol and calcium homeostasis in oxidative hepatocellular injury. Hepatology. 1985 Sep-Oct;5(5):876–882. doi: 10.1002/hep.1840050529. [DOI] [PubMed] [Google Scholar]
- Bright G. R., Fisher G. W., Rogowska J., Taylor D. L. Fluorescence ratio imaging microscopy: temporal and spatial measurements of cytoplasmic pH. J Cell Biol. 1987 Apr;104(4):1019–1033. doi: 10.1083/jcb.104.4.1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis M. H., Altschuld R. A., Jung D. W., Brierley G. P. Estimation of intramitochondrial pCa and pH by fura-2 and 2,7 biscarboxyethyl-5(6)-carboxyfluorescein (BCECF) fluorescence. Biochem Biophys Res Commun. 1987 Nov 30;149(1):40–45. doi: 10.1016/0006-291x(87)91602-0. [DOI] [PubMed] [Google Scholar]
- Emaus R. K., Grunwald R., Lemasters J. J. Rhodamine 123 as a probe of transmembrane potential in isolated rat-liver mitochondria: spectral and metabolic properties. Biochim Biophys Acta. 1986 Jul 23;850(3):436–448. doi: 10.1016/0005-2728(86)90112-x. [DOI] [PubMed] [Google Scholar]
- Grynkiewicz G., Poenie M., Tsien R. Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985 Mar 25;260(6):3440–3450. [PubMed] [Google Scholar]
- Hancock C. C. The beginnings of the American Society of Biological Chemists. Fed Proc. 1987 Feb;46(2):227–229. [PubMed] [Google Scholar]
- Herman B., Nieminen A. L., Gores G. J., Lemasters J. J. Irreversible injury in anoxic hepatocytes precipitated by an abrupt increase in plasma membrane permeability. FASEB J. 1988 Feb;2(2):146–151. doi: 10.1096/fasebj.2.2.3342967. [DOI] [PubMed] [Google Scholar]
- Hoek J. B., Nicholls D. G., Williamson J. R. Determination of the mitochondrial protonmotive force in isolated hepatocytes. J Biol Chem. 1980 Feb 25;255(4):1458–1464. [PubMed] [Google Scholar]
- Johnson L. V., Walsh M. L., Bockus B. J., Chen L. B. Monitoring of relative mitochondrial membrane potential in living cells by fluorescence microscopy. J Cell Biol. 1981 Mar;88(3):526–535. doi: 10.1083/jcb.88.3.526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemasters J. J., DiGuiseppi J., Nieminen A. L., Herman B. Blebbing, free Ca2+ and mitochondrial membrane potential preceding cell death in hepatocytes. Nature. 1987 Jan 1;325(6099):78–81. doi: 10.1038/325078a0. [DOI] [PubMed] [Google Scholar]
- Malgaroli A., Milani D., Meldolesi J., Pozzan T. Fura-2 measurement of cytosolic free Ca2+ in monolayers and suspensions of various types of animal cells. J Cell Biol. 1987 Nov;105(5):2145–2155. doi: 10.1083/jcb.105.5.2145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nayler W. G. Calcium and cell death. Eur Heart J. 1983 May;4 (Suppl 100):33–41. doi: 10.1093/eurheartj/4.suppl_c.33. [DOI] [PubMed] [Google Scholar]
- Poenie M., Alderton J., Steinhardt R., Tsien R. Calcium rises abruptly and briefly throughout the cell at the onset of anaphase. Science. 1986 Aug 22;233(4766):886–889. doi: 10.1126/science.3755550. [DOI] [PubMed] [Google Scholar]
- Scanlon M., Williams D. A., Fay F. S. A Ca2+-insensitive form of fura-2 associated with polymorphonuclear leukocytes. Assessment and accurate Ca2+ measurement. J Biol Chem. 1987 May 5;262(13):6308–6312. [PubMed] [Google Scholar]
- Schanne F. A., Kane A. B., Young E. E., Farber J. L. Calcium dependence of toxic cell death: a final common pathway. Science. 1979 Nov 9;206(4419):700–702. doi: 10.1126/science.386513. [DOI] [PubMed] [Google Scholar]
- Steinberg S. F., Bilezikian J. P., Al-Awqati Q. Fura-2 fluorescence is localized to mitochondria in endothelial cells. Am J Physiol. 1987 Nov;253(5 Pt 1):C744–C747. doi: 10.1152/ajpcell.1987.253.5.C744. [DOI] [PubMed] [Google Scholar]
- Trump B. F., Berezesky I. K., Laiho K. U., Osornio A. R., Mergner W. J., Smith M. W. The role of calcium in cell injury. A review. Scan Electron Microsc. 1980;(Pt 2):437-62, 492. [PubMed] [Google Scholar]