Abstract
The serine/threonine kinase Akt regulates cellular survival, proliferation, gene transcription, protein translation, metabolism, and differentiation. Although Akt substrates are found throughout the cell, activated Akt normally accumulates in the nucleus, suggesting that biologically relevant targets are located there. Consequences of nuclear Akt signaling in cardiomyocytes were explored by using nuclear-targeted Akt (Akt-nuc). Accumulation of Akt-nuc did not provoke hypertrophy, unlike constitutively activated Akt. Instead, Akt-nuc inhibited hypertrophy concurrent with increased atrial natriuretic peptide (ANP) expression that depended upon phosphatidylinositol-3 kinase activity. Akt-nuc antihypertrophic effects were blocked by inhibition of either guanylyl cyclase A receptor or cyclic guanosine monophosphate-dependent protein kinase in cultured cardiomyocytes. Corroborating evidence showed blunted acute hypertrophic remodeling in Akt-nuc transgenic mice after transverse aortic constriction coincident with higher ANP expression and smaller myocyte volume. In addition, Akt-nuc expression improved systolic function and survival in the chronic phase of transverse aortic constriction-induced hypertrophy. Thus, Akt-nuc antagonizes certain aspects of hypertrophy through autocrine/paracrine stimulation of a phosphatidylinositol-3 kinase-dependent signaling cascade that promotes ANP expression, resulting in a unique combination of prosurvival coupled with antihypertrophic signaling.
Keywords: cardiac, signal transduction, nucleus, kinase, remodeling
Akt, also known as protein kinase B, has emerged as a focal point for signal transduction pathways responsible for cell survival (1). Akt is initially activated at the cell membrane in response to stimulation by growth factors such as insulin-like growth factor 1 (IGF-1; ref. 2). After activation, Akt phosphorylates multiple cytoplasmic substrates and translocates to the nucleus, where Akt is thought to regulate gene transcription (3, 4). In cardiomyocytes, Akt prevents apoptosis from ischemia reperfusion (5, 6), volume and/or pressure overload, hypoxia (7), hypoglycemia, or cardiotoxic drugs (8). To exploit this beneficial effect, myocardial Akt signaling has been activated by means of adenoviral gene transfer or transgenic expression of constructs encoding myristoylated or amino acid substituted Akt, as well as phosphatidylinositol-3 kinase (PI3-K), resulting in constitutive Akt activation (5–7, 9–11). However, constitutive Akt activation results in supraphysiological levels of kinase activity in the cytoplasm (12, 13), inducing hypertrophic cardiomyopathy in vivo (9, 10). Constitutive Akt activation induces hypertrophy in cardiomyocytes through phosphorylation of target substrate molecules, including glycogen synthase kinase-3β (14), mammalian target of rapamycin (9), glucose transporter-4 (15), and p70S6-kinase (16), which are predominantly located in the cytoplasm. On the other hand, Akt-associated pathways and substrate proteins leading to antiapoptotic effects and cellular survival are often localized in the nucleus (17–19). However, Akt does not possess an inherent nuclear localization signal sequence, and activated Akt likely depends upon cofactors such as TCL1 (20) or zyxin (21) to promote nuclear localization. To better understand the role of nuclear Akt accumulation in cardiomyocyte signaling and phenotypic effects, we constructed nuclear-targeted Akt (Akt-nuc) with a nuclear localizing signal (22). Expression of Akt-nuc by adenoviral vectors or cardiac-specific transgenesis resulted in protection of cardiomyocytes from apoptotic challenge without concomitant hypertrophy (22). These studies indicate that protective effects of Akt could be dissociated from the hypertrophic phenotype that is often connected to antiapoptotic signaling (23). Thus, Akt-nuc exhibits a unique combination of phenotypic properties with important similarities and differences, in comparison to previous characterizations of Akt activation in cardiomyocytes or the myocardium. Lack of hypertrophic remodeling after Akt-nuc accumulation prompted this investigation to determine the effect of Akt-nuc upon cellular and myocardial responses to hypertrophic stimulation. Most notably in the context of this report, Akt-nuc inhibits hypertrophy and reveals previously unknown phenotypic and molecular effects of Akt signaling.
Results
Akt-nuc Prevents Hypertrophic Remodeling of Cardiomyocytes Treated with Endothelin 1 (ET-1).
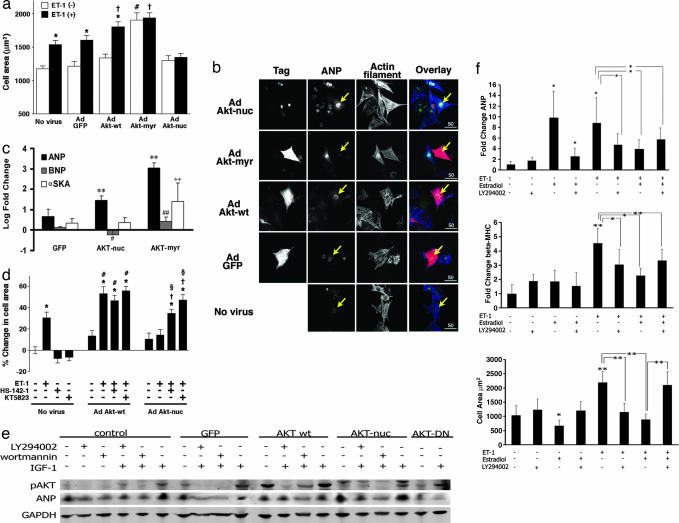
Cardiomyocyte cell area was measured to determine whether accumulation of Akt-nuc causes cardiomyocyte enlargement as reported for myristoylated Akt (Akt-myr), a constitutively activated form of the kinase (5, 7). Expression of Akt-myr significantly enlarged cardiomyocytes compared to cells that were uninfected (162%, P < 0.001; Fig. 1a). No significant differences in cell area were observed between uninfected controls and cardiomyocytes expressing GFP, wild-type Akt (Akt-wt), or Akt-nuc. Treatment with ET-1 induced significant enlargement in cardiomyocytes that were uninfected (131%, P < 0.001) or infected with adenoviruses expressing GFP (132%, P < 0.001) or Akt-wt (135%, P < 0.001). However, cardiomyocytes expressing Akt-nuc did not respond to ET-1 treatment with hypertrophic enlargement (104%, P = 0.6).
Fig. 1.
Akt-nuc inhibits hypertrophy through an ANP-dependent signaling cascade. (a) Akt-nuc inhibits endothelin-induced hypertrophy. Cell surface area measurements are from cardiomyocytes that were either uninfected (No virus) or infected with adenoviruses expressing GFP (Ad GFP), wild-type Akt (Ad Akt-wt), myristoylated Akt (Ad Akt-myr), or nuclear-targeted Akt (Ad Akt-nuc). Each cell population was measured with infection alone (white bars) or infection followed by 48-h stimulation with hypertrophic agonist ET-1 (10−7 mol/liter) (black bars). ∗, P < 0.05 vs. same virus without ET-1. #, P < 0.05 vs. no virus without ET-1. †, P < 0.05 vs. no virus with ET-1. (b) Confocal microscopy of cardiomyocytes stimulated with ET-1 (10−7 mol/liter) indicates that Akt-nuc promotes ANP expression. Antibodies to myc-tag, hemagglutinin-tag, or GFP-tag show infected cells (Tag), whereas ANP expression was detected by anti-ANP antibody (ANP). Tag (red), ANP (green), and actin (blue) are depicted in overlay images. (c) Akt-nuc induces ANP without concomitant increases in α-skeletal actin (αSKA). ANP mRNA transcript levels are significantly increased relative to GFP-expressing control (black bars; ∗∗, P < 0.01). BNP expression shows differential and significant changes between Akt-nuc- and Akt-myr-expressing cells (gray bars; #, P < 0.05; ##, P < 0.01). Expression of hypertrophic marker αSKA is not increased by Akt-nuc expression but is significantly elevated by Akt-myr expression (white bars, ++, P < 0.01). (d) Antihypertrophic effects of Akt-nuc involve autocrine and/or paracrine stimulation of GC-A receptor and PKG. Hypertrophy of cardiomyocytes induced by ET-1 (10−7 mol/liter) was inhibited in Ad Akt-nuc infected cells. Antihypertrophic effects of Ad Akt-nuc were reversed by inhibition of GC-A receptor with HS-142-1 (10 μg/ml) or PKG with KT5823 (10−6 mol/liter). ∗, P < 0.05 vs. no virus without any drugs. #, P < 0.05 vs. Ad Akt-wt without any drugs. †, P < 0.05 vs. Ad Akt-nuc without any drugs. §, P < 0.05 vs. Ad Akt-nuc with ET-1. (e) IGF-mediated induction of ANP depends upon PI3-K/Akt signaling. Immunoblot analysis of cultured cardiomyocyte lysates infected with adenoviral vectors expressing GFP, Akt-wt, Akt-nuc, or Akt-DN proteins. Cultures were subsequently treated the next day with IGF-1 alone or in combination with PI3-K inhibitors LY294002 or wortmannin. (f) Antihypertrophic effect of estradiol is abrogated by inhibition of PI3-K. Cultured cardiomyocytes were exposed to estradiol alone or in combination with LY294002 and subsequently stimulated by ET-1 treatment. ∗, P < 0.05; ∗∗, P < 0.01 for each comparison indicated. See Supporting Text, which is published as supporting information on the PNAS web site, for expanded detailed information of this figure.
Akt-nuc Specifically Induces ANP Gene Expression in Vitro.
Atrial natriuretic peptide (ANP) localization was analyzed in the presence of ET-1 by immunostaining (Fig. 1b). ET-1 stimulation resulted in perinuclear ANP staining in uninfected cells (No virus, arrow). Levels of perinuclear ANP staining in cardiomyocytes expressing GFP (Ad GFP, arrow) and Akt-wt (Ad Akt-wt, arrow) were comparable to that of uninfected cells. However, perinuclear ANP staining in cardiomyocytes expressing Akt-nuc (Ad Akt-nuc, arrow) was more intense than that of uninfected cells.
ANP mRNA transcript levels in cardiomyocytes infected with adenoviral vectors for GFP, AKT-nuc, or AKT-myr was measured by real-time RT-PCR together with B-type natriuretic peptide (BNP) and the cytoskeletal hypertrophic marker α-skeletal actin (α-SKA; Fig. 1c). ANP was induced by AKT-nuc (28.9-fold; P = 0.004) and myr-AKT (1,098-fold; P = 0.0008) vs. GFP-infected cells. High-level ANP induction by AKT-myr likely reflects constitutive activation of this protein, leading to exaggerated hypertrophic stimulation in combination with nuclear accumulation from overexpression and chronic activation (12, 24). In comparison, BNP was induced 2.4-fold by AKT-myr (P = 0.009) but was significantly decreased upon AKT-nuc infection (0.5-fold; P = 0.046). Notably, α-SKA transcript levels showed the most profound difference, with 20.98-fold induction by AKT-myr (P < 0.0001) but unchanged by AKT-nuc infection vs. GFP control. These results demonstrate that AKT-nuc exhibits a distinct transcriptional profile characterized by induction of ANP without concomitant increases in α-SKA, as noted for AKT-myr expression.
Antagonizing Effect of Akt-nuc upon ET-1 Stimulation Is Guanylyl Cyclase A (GC-A) Receptor Protein Kinase G (PKG)-Dependent.
Participation of ANP in mediating antihypertrophic effects of Akt-nuc was supported by experiments by using HS-142–1 (a GC-A receptor antagonist) and KT5823 [a specific cyclic guanosine monophosphate (cGMP)-dependent PKG inhibitor; Fig. 1d]. ET-1 stimulation increased the mean cell area of uninfected cells by 30.6%. ET-1 stimulation significantly increased mean cell area of cardiomyocytes expressing Akt-wt by 53.2% compared to uninfected cells or cells expressing Akt-wt without ET-1 stimulation (P < 0.05), and this increase in mean cell area was unaffected by either HS-142–1 or KT5823 treatment. The mean cell area of cardiomyocytes expressing Akt-nuc was not increased by ET-1 stimulation, but the ability of Akt-nuc to blunt hypertrophy under ET-1 stimulation was lost when cardiomyocytes were exposed to either HS-142–1 or KT5823, implicating ANP-receptor and cGMP-dependent signaling in mitigation of hypertrophy by Akt-nuc.
Induction of ANP Protein Expression Correlates with the PI3-K/Akt Signaling Cascade.
The relationship between ANP production and the PI3-K/Akt signaling pathway activity was verified in cardiomyocytes infected with adenoviral vectors expressing GFP, Akt-wt, Akt-nuc, or dominant-negative Akt (Akt-DN) proteins in conjunction with exposure to IGF-1 alone or together with PI3-K inhibitors (LY294002 or wortmannin). IGF-1-mediated induction of ANP protein expression correlates with phospho-Akt473 accumulation that was blocked by PI3-K inhibition, whereas Akt-wt overexpression induces phospho-Akt473 accumulation and a moderate production of ANP that appears unperturbed by PI3-K inhibition. In comparison, Akt-nuc promotes robust ANP protein accumulation that is blunted by PI3-K inhibition. Lastly, Akt-DN overexpression blocks IGF-1-induced ANP production, clearly demonstrating a connection between Akt activity and the production of ANP protein.
Estradiol-Mediated Inhibition of Hypertrophy Involves PI3-K-Dependent Signaling.
Endogenous PI3-K-dependent signaling to inhibit hypertrophy was examined in cardiomyocytes pretreated with 30 μM LY294002 for 1 h, then 10−7 M estradiol for 3 h, and ultimately stimulated with 10−7 M endothelin for 16 h (Fig. 1f). Nuclear accumulation of phospho-Akt473 after estradiol stimulation inhibited by LY294002 was confirmed by confocal microscopy (data not shown). Real-time RT-PCR quantitation of mRNA levels for ANP and β-myosin heavy chain (β-MHC) reveals significant ANP induction with estradiol treatment (9.7-fold, P = 0.03) that was reversed by LY294002 (P = 0.03). ANP induction mediated by ET-1 (8.8-fold, P = 0.03) was also inhibited by LY294002 (P = 0.03) as well as estradiol (P = 0.03). Inhibition of ANP production by estradiol treatment of ET-1-stimulated cultures was partially reversed by addition of LY294002 (5.7-fold increase, P = 0.02 vs. estradiol + ET, and 0.02 vs. ET-1 alone). In comparison, induction of β-MHC by ET-1 (4.5-fold, P = 0.0026) was inhibited by LY294002 (3.0-fold, P = 0.013) and estradiol (2.26-fold, P = 0.010). The inductive effect of ET-1 upon β-MHC in estradiol-treated cells was partially restored upon exposure to LY294002 (3.3-fold, P = 0.061 vs. ET-1 alone). These findings of β-MHC regulation correlate with measurements of cell area. Quantitation of cell area by confocal microscopy shows significant inhibition of ET-1-induced cellular hypertrophy by estradiol (P = 0.005) with significant restoration of hypertrophic remodeling after addition of LY294002 (P = 0.04). Despite inhibition of hypertrophic remodeling by Akt-nuc infection of cultured cardiomyocytes, measurements of protein synthesis rate and protein content were comparable with control cultures (results not shown). We attribute this observation to additional effects of Akt-nuc in cultured cardiomyocytes that enhance protein accumulation by hypertrophy-independent mechanisms, including enhanced resistance to cell death as well as increased proliferation in vitro. In fact, despite comparable protein content in both cultures, estradiol-treated cells are significantly smaller than ET-1-treated cells (Fig. 1f Bottom). Thus, we relied upon the combination of 2D cell area measurements with transcriptional changes to track hypertrophic effects in our cultured cardiomyocytes. These experiments indicate that the ability of estradiol to inhibit hypertrophy and induce ANP transcription depends in part upon PI3-K activity.
Akt-nuc Improves Chronic Survival After Myocardial Pressure Overload.
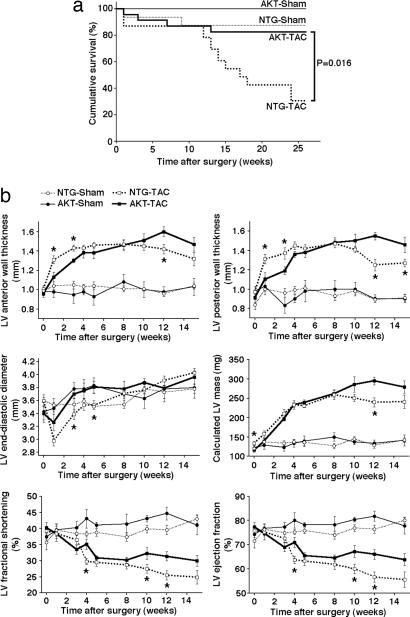
Weights of the body, heart, lung, and liver were comparable between cardiac-specific Akt-nuc transgenic mice (AKT) and nontransgenic (NTG) age- and gender-matched controls (Table 1, which is published as supporting information on the PNAS web site). Myocardial pressure overload was induced by transverse aortic constriction (TAC). NTG mice with TAC surgery (NTG-TAC) succumb to cardiomyopathic effects beginning at 12 wk (Fig. 2a). In comparison, AKT mice with TAC surgery (AKT-TAC) show significantly better survival (P = 0.016). TUNEL assays performed on samples 14 wk after TAC show comparable levels of apoptosis in both NTG-TAC and AKT-TAC group, suggesting that differences in apoptotic cell death did not contribute significantly to enhanced AKT-TAC survival. However, measurements of ongoing cell death at death do not exclude that differences in apoptosis were present in the two groups of animals earlier during the evolution of pressure-overload hypertrophy.
Fig. 2.
Survival is improved and remodeling is blunted in Akt-nuc mouse hearts after TAC. (a) Improved chronic survival of Akt-nuc transgenic (AKT) mice after TAC. Kaplan–Meier survival plots for NTG TAC (NTG-TAC, n = 23), AKT-TAC (n = 23), NTG-sham (n = 16), and AKT-sham (n = 11) revealed that AKT-TAC have significantly better survival rates than NTG-TAC. (b) Serial echocardiographic measurements of NTG-TAC (n = 15), AKT-TAC (n = 8), NTG-sham (n = 8), and AKT-sham (n = 4) mice revealed that AKT-TAC mice have blunted remodeling of LV anterior and posterior wall thickness in acute phase (1–3 wk after TAC) and improved LV fractional shortening and LV ejection fraction in chronic phase (10 wk after TAC and later) than NTG-TAC mice. ∗, P < 0.05 vs. AKT-TAC.
Akt-nuc Antagonizes Cardiac Hypertrophy After Myocardial Pressure Overload.
Myocardial remodeling in AKT and NTG mice subjected to TAC surgery was assessed by serial echocardiographic measurements (Fig. 2b). In the acute phase, defined as 1–3 wk after TAC, AKT-TAC hearts showed significantly blunted increases in left ventricular (LV) anterior and posterior wall thickness relative to NTG-TAC (P < 0.05). LV end-diastolic diameter of AKT-TAC at 3 and 5 wk after TAC was significantly larger than NTG-TAC (P < 0.05). LV fractional shortening and LV ejection fraction were comparable between AKT-TAC and NTG-TAC in the acute phase. In the chronic phase, defined as 10 wk after TAC and later, AKT-TAC showed significantly improved LV fractional shortening (P < 0.05) and LV ejection fraction (P < 0.05) relative to NTG-TAC.
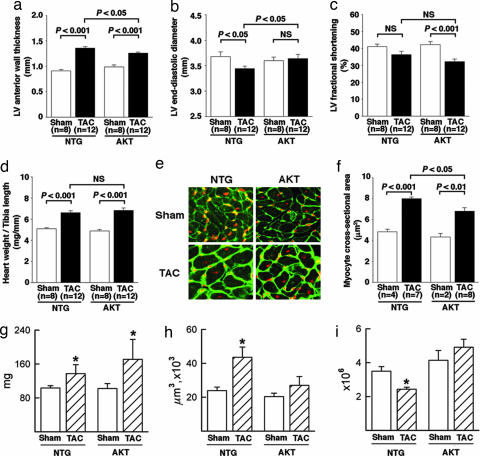
Echocardiographic (Fig. 3a–c) and postmortem (Fig. 3 d–f) measurements taken 2 wk after TAC surgery reveal decreased ventricular wall thickness (Fig. 3a, P < 0.05) and increased LV end-diastolic diameter (Fig. 3b, P < 0.05) in AKT-TAC compared to NTG-TAC. Comparable values for both AKT-TAC and NTG-TAC were observed for LV fractional shortening (Fig. 3c) and heart weight to tibia length ratio (Fig. 3d). Measurement of cardiomyocyte cross-sectional area sampled from heart sections (Fig. 3e) together with quantitative analysis (Fig. 3f) revealed a smaller cross-sectional area of cells in AKT-TAC compared to those in NTG-TAC (P < 0.05). Morphometric analyses of chronic phase hearts at 14 wk after TAC for LV mass (Fig. 3g), cell volume of LV myocytes (Fig. 3h), and number of LV myocytes (Fig. 3i) reveal that hearts of AKT-TAC mice are significantly heavier than those of NTG controls at this late time point (P < 0.05). However, this increase in mass cannot be accounted for by cell volume increases, which are actually less in AKT-TAC than NTG-TAC. Calculations reveal that, whereas NTG-TAC cardiomyocytes increase in volume 1.81-fold relative to sham-operated NTG controls, the AKT-TAC cardiomyocytes enlarge only 1.32-fold relative to sham-operated AKT controls. Instead, the basis for increased mass of the AKT-TAC myocardium results from the hypercellular phenotype of the transgenic heart coupled with a lower rate of myocyte loss. Whereas fewer larger myocytes were present at 14 wk after TAC in the NTG LV, myocytes in the Akt-nuc transgenic LV showed no significant change in either volume (Fig. 3h) or number (Fig. 3i).
Fig. 3.
Echocardiographic and postmortem analysis of mice at 2 wk after TAC reveals inhibition of concentric hypertrophy and smaller cross-sectional cell area in AKT-TAC relative to NTG-TAC hearts. (a) LV anterior wall thickness. (b) LV end-diastolic diameter. (c) LV fractional shortening. (d) heart weight/tibia length in NTG-sham (n = 8), NTG-TAC (n = 12), AKT-sham (n = 8), and AKT-TAC (n = 12) at 2 wk after surgery. (e) representative heart sections from sham or TAC of NTG or AKT mice. Cell membrane (green) and nucleus (red) were labeled for analyses, with measurements limited to only those cells with a clear nucleus surrounded by cell membrane. (f) Quantitative analysis of myocyte cross-sectional area in NTG-sham (n = 4), NTG-TAC (n = 7), AKT-sham (n = 3), and AKT-TAC (n = 8) mice. Cross-sectional area of 100 cells was measured in the LV-free walls of each animal. Calculations of LV mass (g), myocyte volume (h), and myocyte number (i) from mice 14 wk after TAC based on morphometric analyses of heart as described in Methods. LV mass is significantly increased in both NTG and AKT hearts in the chronic remodeling phase with cell volume of myocytes increasing significantly in the NTG-TAC but not in the AKT-TAC hearts. Myocyte number in the AKT-TAC heart is increased relative to the NTG-TAC, indicating that AKT-TAC myocardium possesses more numerous but smaller myocytes. n = 5 for all morphometric calculation groups except for NTG-TAC, where n = 3 because of increased mortality. ∗, P < 0.05 for comparisons between paired sets of samples as shown.
Akt-nuc Specifically Induces ANP Gene Expression in Pressure Overload Model.
Differences in mRNA transcript levels for ANP, BNP, c-for, β-MHC, and α-SKA between NTG-TAC and AKT-TAC mice were assessed at 1 wk after surgery (Fig. 4a). ANP expression was increased to a higher level in AKT-TAC hearts relative to NTG-TAC (26- vs. 10-fold, P = 0.003), whereas BNP was significantly less elevated in AKT-TAC than NTG-TAC (5.3- vs. 9.1-fold, P = 0.0015). Insignificant differences between NTG-TAC and AKT-TAC expression levels were found for both β-MHC and c-fos. Notably, α-SKA was elevated in both groups vs. their sham controls but to a significantly lesser degree in AKT-TAC relative to NTG-TAC (3.56- vs. 8.7-fold; P = 0.0154). No significant differences for any transcripts examined were noted between sham-operated NTG vs. AKT (Fig. 4b). Subsequent immunoblot of cardiac lysates at 1 wk after TAC confirmed significantly increased ANP protein expression in AKT-TAC relative to NTG-TAC as shown (Fig. 4c; P < 0.05) with quantitative densitometry for ANP protein levels normalized to GAPDH protein (Fig. 4d). Thus, Akt-nuc promotes ANP expression in response to pressure overload challenge and exhibits selective repression hypertrophic reprogramming by inhibiting α-SKA but not β-MHC.
Fig. 4.
ANP expression is increased in AKT-TAC relative to NTG-TAC mice at 1 wk after TAC surgery. (a) Transcript levels for ANP, BNP, c-fos, β-MHC, and α-SKA were quantified by real-time RT-PCR in mRNA samples. Fold change in abundance is presented after standardization to GAPDH as an internal control. ANP mRNA transcript levels are significantly increased in AKT-TAC (gray bars) relative to NTG-TAC (black bars) samples (∗∗, P < 0.01). In contrast, transcript levels of BNP and α-SKA were significantly decreased in AKT-TAC relative to NTG-TAC samples (∗∗, P < 0.01; ∗, P < 0.05). (b) Changes in mRNA transcript levels represented as fold change in sham-operated AKT relative to NTG myocardial samples probed for ANP, BNP, c-fos, β-MHC, and α-SKA by real-time RT-PCR. Fold change in abundance is presented after standardization to GAPDH as an internal control. No significant differences were observed between AKT and NTG samples before TAC challenge. (c) Cardiac lysates at 1 wk after surgery were examined for protein expression of ANP, myc-tag, and Akt by immunoblot analyses with GAPDH and were included to standardize for minor variations in loading between samples. (d) Quantitative densitometry of ANP relative to GAPDH protein levels in NTG-sham, NTG-TAC, AKT-sham, and AKT-TAC mice.
Discussion
The ability of Akt kinase to regulate critical cellular processes such as metabolism, hypertrophy, and survival prompted numerous studies of Akt-mediated effects both in vitro (5, 7) and in vivo (9–11, 25, 26). Studies of Akt myocardial biology involving overexpression have been predominantly conducted with constitutively activated forms of the kinase created by molecular engineering (5, 7, 9, 11, 25). Characterizing constitutive Akt activation in the myocardium produces two recurring themes: promotion of growth and antiapoptotic activity. Promotion of growth is observed in vivo, particularly in the assessment of postnatal cardiac enlargement resulting from transgenic expression of Akt kinase in the myocardium (9, 10, 25) and in the hypertrophic growth of cardiomyocytes in vitro (9, 10, 25). Antiapoptotic effects are a consistent finding both in vitro (5, 7) and in vivo (9, 11). These studies have substantially furthered our understanding of Akt-mediated signaling and remodeling, but they may not accurately reflect the physiologic actions of Akt activation. Acute effects of Akt with constitutive activation are cardioprotective, but chronic Akt activation renders the myocardium more susceptible to ischemia-reperfusion injury (27). Alternatively, IGF-1 also activates Akt in the myocardium (28) but exhibits phenotypic characteristics of apoptotic resistance and hypercellularity after cardiac-specific overexpression (29). Additional multiple aspects of cardiac-specific constitutively activated Akt overexpression resulting in cardiac hypertrophy (9, 10, 25), depressed contractile function (9), and dilatation (10) are not readily reconciled with the phenotype of cardiac-specific IGF-1 transgenic overexpression. One potential explanation for these phenotypic distinctions between constitutively activated Akt- and IGF-1 overexpression in the heart may be the degree and distribution of Akt activity, prompting our investigation into the consequences resulting from targeted activation of Akt in the nucleus of cardiomyocytes.
Accumulation of Akt-nuc did not provoke hypertrophic enlargement in the present study but instead inhibited ET-1-induced cardiomyocyte hypertrophy in cultured cells with concurrent increases in ANP expression. Activation of Akt is predominantly responsible for β-adrenergic receptor-stimulated ANP transcription (30). Furthermore, Akt activity antagonizes hairy-related transcription factor-mediated effects that, in turn, inhibit GATA-dependent transcriptional activation (31). Because GATA factors enhance expression of cardiac-associated promoters, including ANP (32), it is reasonable to postulate that Akt activation enhances ANP production. The antihypertrophic effect of Akt-nuc was blocked by inhibition of either GC-A receptor or PKG signaling. HS-142–1 and KT5823 inhibitors used in this study have demonstrated participation of GC-A receptor-PKG signaling in ANP-mediated protection (21). Antihypertrophic effects of Akt-nuc were abrogated by treatment of cultures with HS-142–1 or KT5823 before ET-1 stimulation, confirming involvement of GC-A receptor-mediated or cGMP-dependent signaling in Akt-nuc-mediated antihypertrophic effects, respectively. These findings indicate that Akt-nuc antagonizes hypertrophy in part, although promotion of autocrine/paracrine stimulation that operates by a GC-A receptor–PKG-dependent pathway. In concordance with this postulate, cardiac-specific Akt-nuc expression increases ANP expression in ventricles of mice subjected to pressure overload compared with NTG mice. Collectively, our results implicate ANP as one of the effectors responsible for the antihypertrophic effect of Akt-nuc, especially because the antihypertrophic properties of ANP are well documented (33). However, prosurvival and proproliferative effects of Akt-nuc leading to a hypercellular myocardium result in a novel constellation of selected antihypertrophic effects upon cardiomyocytes distinct from ANP/PKG-mediated antihypertrophic signaling. Although ANP has been shown to be antihypertrophic, it is not a “kill switch” to inhibit remodeling. Rather, it is a brake on the hypertrophic process, and the participation of nuclear Akt in antagonizing hypertrophy appears to be mediated at least in part by potentiating ANP expression.
17β-estradiol activates Akt (34) and antagonizes cardiac hypertrophy by inducing ANP in vitro (35), as well as blunting pressure-overload hypertrophy in vivo (36). Furthermore, we reported a correlation between nuclear-localized phospho-Akt473 and the presence of estrogenic stimulation in samples of human myocardium by immunohistochemical analyses as well as in cultured neonatal rat cardiomyocyte after exposure to 17β- estradiol (37). Thus, together with our estradiol results (Fig. 1f), it is tempting to speculate that 17β-estradiol antagonizes cardiac hypertrophy by activating Akt that, in turn, increases ANP production.
Multiple concurrent processes that may occur in response to Akt activation contribute to altered hypertrophy in addition to the ANP-related effects examined in this study. As we previously reported, nuclear-Akt-mediated signaling events are associated with activation of PKG and cGMP (21) in addition to published studies linking Akt activation to nitric oxide-dependent signaling (38, 39). Implicit in these intimate signaling interrelationships is the likelihood that alteration of any facet of the cascade will have repercussions for hypertrophic remodeling. Additionally, Akt-nuc transgenic mice exhibit enhanced contractile function and resistance to apoptotic cell death that could influence signal transduction and remodeling in the early phase of pressure overload challenge (22, 40). Current studies ongoing in the lab use PKG-expressing recombinant adenoviruses, transgenic mice with cardiac-specific expression of guanylyl cyclase to increase cGMP production, and multiple pharmacologic inhibitors to tease apart the respective contributions of these pathways in the remodeling process.
Several concurrent mechanisms are likely to account for the emergence of late-stage hypertrophy in the transgenic nuclear Akt mice. First, altered ANP production in the chronic hypertrophic phase may be further exacerbated by receptor down-regulation/desensitization observed in heart failure (41). Also, we previously reported that the therapeutic threshold of ANP is a relatively narrow window, and that excessive levels of ANP promote cardiomyocyte apoptosis (21). Thus, altered ANP signaling in the chronic phase of our TAC model potentially contributes to enhanced remodeling. An equally plausible explanation is the predominance of alternate hypertrophic signaling later stages of pressure-overload-induced remodeling and cardiac failure, but such mechanisms are beyond the scope of the present study focused upon the antihypertrophic actions of nuclear Akt. In the late stage of response to TAC >3 mo after challenge, ventricular mass was elevated in the AKT-TAC mice, and this appears to be due to trends toward increased number of myocytes with modest cellular hypertrophy. Although neither of these two changes was statistically significant, together they resulted in significant expansion in the muscle compartment of the myocardium.
In conclusion, Akt-nuc antagonizes apoptosis, induces ANP expression, and antagonizes cardiac hypertrophy. Potent survival signaling in combination with antagonizing hypertrophic signaling makes Akt-nuc an interesting candidate for therapeutic intervention against cardiomyopathic injuries resulting from ischemia reperfusion, chronic pressure or volume overload, hypoxia, or anticancer drugs.
Methods
Cardiomyocyte Preparation, Stimulation, Inhibitory Treatments, and Adenoviral Infections.
Neonatal rat ventricular myocytes were isolated as described (22). Adenoviruses encoding GFP, Akt-wt, Akt-myr, and Akt-nuc were prepared and used as described (21, 22). Additional details are provided in Supporting Text.
Confocal Microscopy, Histology, and Morphometric Analyses.
Cardiomyocytes cultured in two-well chamber slides were rinsed in PBS and fixed in 4% paraformaldehyde. Cardiomyocytes were immunostained with mouse anti-myc tag (Cell Signaling Technology; 1:100 dilution) or mouse anti-hemagglutinin (HA) tag (Cell Signaling Technology; 1:100 dilution) antibody to identify cells infected with Akt-wt, Akt-nuc, or Akt-myr. ANP was immunostained with rabbit anti-ANP antibody (Peninsula Laboratories; 1:100 dilution). The next day, slides were washed three times for 10 min in PBS and incubated 1 h at room temperature in the dark with FITC-conjugated donkey anti-mouse IgG and Cy5-conjugated donkey anti-rabbit IgG antibody (Jackson ImmunoResearch) diluted 1:100 in blocking solution. Simultaneous staining with Texas red-conjugated phalloidin (Molecular Probes; 1:100 dilution) revealed actin filaments. After secondary labeling, slides were washed three times in PBS for 5 min per wash and mounted for viewing in Vectashield medium (Vector Laboratories). Details of procedures for labeling cultured cells as well as histological and morphometric analysis information are provided in Supporting Text.
Real-Time Quantitative RT-PCR and Biochemical Analyses.
Total RNA was extracted from the heart tissues or cultured cardiomyocytes by using TRIzol reagent (Invitrogen) according to the manufacturer’s instructions. To avoid genomic DNA contamination, DNA degradation was performed by using RQ1 RNase-Free DNase (Promega) according to the manufacturer’s instructions. Complementary DNAs (cDNAs) were synthesized by standard techniques by using the SuperScript III First-Strand Synthesis System for RT-PCR (Invitrogen) with oligo(dT) primers. Details of procedures for RT-PCR including primer sets, gel electrophoresis, and immunoblot detection of samples are provided in Supporting Text.
Mice, Transverse Aortic Constriction Surgery, Echocardiographic Analyses, and Statistical Analyses.
Transgenic AKT mice have been described (22). Age-matched NTG were used as controls. All experimental protocols were approved by the San Diego State University Institutional Animal Care and Use Committee. Surgical procedures, echocardiography, and statistical analyses are detailed in Supporting Text.
Supplementary Material
Acknowledgments
This work was supported by National Institutes of Health Grants HL58224, HL66035, and HL67245 (to M.A.S.) and P01AG023071 (to P.A.). M.A.S. is an Established Investigator of the American Heart Association (0040051N). J.M. is a Fellow of the Rees–Stealy Research Foundation and the San Diego State University Heart Institute.
Abbreviations
- Akt-nuc
nuclear-targeted Akt
- Akt-wt
wild-type Akt
- Akt-myr
myristoylated Akt
- ANP
atrial natriuretic peptide
- GC-A
guanylyl cyclase A
- PKG
protein kinase G
- TAC
transverse aortic constriction
- ET-1
endothelin 1
- AKT
cardiac-specific Akt-nuc transgenic mice
- NTG
nontransgenic control mice
- α-SKA
α-skeletal actin
- β-MHC
β-myosin heavy chain
- IGF-1
insulin-like growth factor 1
- BNP
B-type natriuretic peptide
- PI3-K
phosphatidylinositol-3 kinase
- cGMP
cyclic guanosine monophosphate
- LV
left ventricular
- NTG-TAC
NTG with TAC surgery
- AKT-TAC
AKT with TAC surgery.
Footnotes
Conflict of interest statement: No conflicts declared.
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Datta S. R., Brunet A., Greenberg M. E. Genes Dev. 1991;13:2905–2927. doi: 10.1101/gad.13.22.2905. [DOI] [PubMed] [Google Scholar]
- 2.Alessi D. R., Andjelkovic M., Caudwell B., Cron P., Morrice N., Cohen P., Hemmings B. A. EMBO J. 1996;15:6541–6551. [PMC free article] [PubMed] [Google Scholar]
- 3.Brazil D. P., Park J., Hemmings B. A. Cell. 2002;111:293–303. doi: 10.1016/s0092-8674(02)01083-8. [DOI] [PubMed] [Google Scholar]
- 4.Andjelkovic M., Alessi D. R., Meier R., Fernandez A., Lamb N. J., Frech M., Cron P., Cohen P., Lucocq J. M., Hemmings B. A. J. Biol. Chem. 1997;272:31515–31524. doi: 10.1074/jbc.272.50.31515. [DOI] [PubMed] [Google Scholar]
- 5.Fujio Y., Nguyen T., Wencker D., Kitsis R. N., Walsh K. Circulation. 2000;101:660–667. doi: 10.1161/01.cir.101.6.660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Miao W., Luo Z., Kitsis R. N., Walsh K. J. Mol. Cell. Cardiol. 2000;32:2397–2402. doi: 10.1006/jmcc.2000.1283. [DOI] [PubMed] [Google Scholar]
- 7.Matsui T., Li L., del Monte F., Fukui Y., Franke T. F., Hajjar R. J., Rosenzweig A. Circulation. 1999;100:2373–2379. doi: 10.1161/01.cir.100.23.2373. [DOI] [PubMed] [Google Scholar]
- 8.Negoro S., Oh H., Tone E., Kunisada K., Fujio Y., Walsh K., Kishimoto T., Yamauchi-Takihara K. Circulation. 2001;103:555–561. doi: 10.1161/01.cir.103.4.555. [DOI] [PubMed] [Google Scholar]
- 9.Shioi T., McMullen J. R., Kang P. M., Douglas P. S., Obata T., Franke T. F., Cantley L. C., Izumo S. Mol. Cell. Biol. 2002;22:2799–2809. doi: 10.1128/MCB.22.8.2799-2809.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Matsui T., Li L., Wu J. C., Cook S. A., Nagoshi T., Picard M. H., Liao R., Rosenzweig A. J. Biol. Chem. 2002;277:22896–22901. doi: 10.1074/jbc.M200347200. [DOI] [PubMed] [Google Scholar]
- 11.Matsui T., Tao J., del Monte F., Lee K. H., Li L., Picard M., Force T. L., Franke T. F., Hajjar R. J., Rosenzweig A. Circulation. 2001;104:330–335. doi: 10.1161/01.cir.104.3.330. [DOI] [PubMed] [Google Scholar]
- 12.Ahmed N. N., Franke T. F., Bellacosa A., Datta K., Gonzalez-Portal M. E., Taguchi T., Testa J. R., Tsichlis P. N. Oncogene. 1993;8:1957–1963. [PubMed] [Google Scholar]
- 13.Aoki M., Batista O., Bellacosa A., Tsichlis P., Vogt P. K. Proc. Natl. Acad. Sci. USA. 1998;95:14950–14955. doi: 10.1073/pnas.95.25.14950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cross D. A., Alessi D. R., Cohen P., Andjelkovich M., Hemmings B. A. Nature. 1995;378:785–789. doi: 10.1038/378785a0. [DOI] [PubMed] [Google Scholar]
- 15.Ueki K., Yamamoto-Honda R., Kaburagi Y., Yamauchi T., Tobe K., Burgering B. M., Coffer P. J., Komuro I., Akanuma Y., Yazaki Y., et al. J. Biol. Chem. 1998;273:5315–5322. doi: 10.1074/jbc.273.9.5315. [DOI] [PubMed] [Google Scholar]
- 16.Sadoshima J., Izumo S. Circ. Res. 1995;77:1040–1052. doi: 10.1161/01.res.77.6.1040. [DOI] [PubMed] [Google Scholar]
- 17.Biggs W. H., III, Meisenhelder J., Hunter T., Cavenee W. K., Arden K. C. Proc. Natl. Acad. Sci. USA. 1999;96:7421–7426. doi: 10.1073/pnas.96.13.7421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kops G. J., de Ruiter N. D., De Vries-Smits A. M., Powell D. R., Bos J. L., Burgering B. M. Nature. 1999;398:630–634. doi: 10.1038/19328. [DOI] [PubMed] [Google Scholar]
- 19.Du K., Montminy M. J. Biol. Chem. 1998;273:32377–32379. doi: 10.1074/jbc.273.49.32377. [DOI] [PubMed] [Google Scholar]
- 20.Pekarsky Y., Koval A., Hallas C., Bichi R., Tresini M., Malstrom S., Russo G., Tsichlis P., Croce C. M. Proc. Natl. Acad. Sci. USA. 2000;97:3028–3033. doi: 10.1073/pnas.040557697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kato T., Muraski J., Chen Y., Tsujita Y., Wall J., Glembotski C. C., Schaefer E., Beckerle M., Sussman M. A. J. Clin. Invest. 2005;115:2716–2730. doi: 10.1172/JCI24280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shiraishi I., Melendez J., Ahn Y., Skavdahl M., Murphy E., Welch S., Schaefer E., Walsh K., Rosenzweig A., Torella D., et al. Circ. Res. 2004;94:884–891. doi: 10.1161/01.RES.0000124394.01180.BE. [DOI] [PubMed] [Google Scholar]
- 23.van Empel V. P., De Windt L. J. Cardiovasc. Res. 2004;63:487–499. doi: 10.1016/j.cardiores.2004.02.013. [DOI] [PubMed] [Google Scholar]
- 24.Gupta D., Syed N. A., Roesler W. J., Khandelwal R. L. J. Cell Biochem. 2004;93:513–525. doi: 10.1002/jcb.20200. [DOI] [PubMed] [Google Scholar]
- 25.Condorelli G., Drusco A., Stassi G., Bellacosa A., Roncarati R., Iaccarino G., Russo M. A., Gu Y., Dalton N., Chung C., et al. Proc. Natl. Acad. Sci. USA. 2002;99:12333–12338. doi: 10.1073/pnas.172376399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim Y. K., Kim S. J., Yatani A., Huang Y., Castelli G., Vatner D. E., Liu J., Zhang Q., Diaz G., Zieba R., et al. J. Biol. Chem. 2003;278:47622–47628. doi: 10.1074/jbc.M305909200. [DOI] [PubMed] [Google Scholar]
- 27.Nagoshi T., Matsui T., Aoyama T., Leri A., Anversa P., Li L., Ogawa W., del Monte F., Gwathmey J. K., et al. J. Clin. Invest. 2005;115:2128–2138. doi: 10.1172/JCI23073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yamashita K., Kajstura J., Discher D. J., Wasserlauf B. J., Bishopric N. H., Anversa P., Webster K. A. Circ. Res. 2001;88:609–614. doi: 10.1161/01.res.88.6.609. [DOI] [PubMed] [Google Scholar]
- 29.Reiss K., Cheng W., Ferber A., Kajstura J., Li P., Li B., Olivetti G., Homcy C. J., Baserga R., Anversa P. Proc. Natl. Acad. Sci. USA. 1996;93:8630–8635. doi: 10.1073/pnas.93.16.8630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Morisco C., Zebrowski D., Condorelli G., Tsichlis P., Vatner S. F., Sadoshima J. J. Biol. Chem. 2000;275:14466–14475. doi: 10.1074/jbc.275.19.14466. [DOI] [PubMed] [Google Scholar]
- 31.Kathiriya I. S., King I. N., Murakami M., Nakagawa M., Astle J. M., Gardner K. A., Gerard R. D., Olson E. N., Srivastava D., Nakagawa O. J. Biol. Chem. 2004;279:54937–54943. doi: 10.1074/jbc.M409879200. [DOI] [PubMed] [Google Scholar]
- 32.Grepin C., Dagnino L., Robitaille L., Haberstroh L., Antakly T., Nemer M. Mol. Cell. Biol. 1994;14:3115–3129. doi: 10.1128/mcb.14.5.3115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Horio T., Nishikimi T., Yoshihara F., Matsuo H., Takishita S., Kangawa K. Hypertension. 2000;35:19–24. doi: 10.1161/01.hyp.35.1.19. [DOI] [PubMed] [Google Scholar]
- 34.Patten R. D., Pourati I., Aronovitz M. J., Baur J., Celestin F., Chen X., Michael A., Haq S., Nuedling S., Grohe C., et al. Circ. Res. 2004;95:692–699. doi: 10.1161/01.RES.0000144126.57786.89. [DOI] [PubMed] [Google Scholar]
- 35.Babiker F. A., De Windt L. J., van Eickels M., Thijssen V., Bronsaer R. J., Grohe C., van Bilsen M., Doevendans P. A. Circulation. 2004;109:269–276. doi: 10.1161/01.CIR.0000105682.85732.BD. [DOI] [PubMed] [Google Scholar]
- 36.van Eickels M., Grohe C., Cleutjens J. P., Janssen B. J., Wellens H. J., Doevendans P. A. Circulation. 2001;104:1419–1423. doi: 10.1161/hc3601.095577. [DOI] [PubMed] [Google Scholar]
- 37.Camper-Kirby D., Welch S., Walker A., Shiraishi I., Setchell K. D., Schaefer E., Kajstura J., Anversa P., Sussman M. A. Circ. Res. 2001;88:1020–1027. doi: 10.1161/hh1001.090858. [DOI] [PubMed] [Google Scholar]
- 38.Calderone A., Thaik C. M., Takahashi N., Chang D. L., Colucci W. S. J. Clin. Invest. 1998;101:812–818. doi: 10.1172/JCI119883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gao F., Gao E., Yue T. L., Ohlstein E. H., Lopez B. L., Christopher T. A., Ma X. L. Circulation. 2002;105:1497–1502. doi: 10.1161/01.cir.0000012529.00367.0f. [DOI] [PubMed] [Google Scholar]
- 40.Rota M., Boni A., Urbanek K., Padin-Iruegas E., Kajstura J., Fiore G., Kudo H., Sonnenblick E. H., Musso E., Houser S. R., et al. Circ. Res. 2005;97:1332–1341. doi: 10.1161/01.RES.0000196568.11624.ae. [DOI] [PubMed] [Google Scholar]
- 41.Tsutamoto T., Kanamori T., Morigami N., Sugimoto Y., Yamaoka O., Kinoshita M. Circulation. 1993;87:70–75. doi: 10.1161/01.cir.87.1.70. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.