Abstract
Mutation of the transcription factor and tumor suppressor gene WT1 results in a range of genitourinary anomalies in humans, including 46,XY gonadal dysgenesis, indicating that WT1 plays a critical role in sex determination. However, because knockout of Wt1 in mice results in apoptosis of the genital ridge, it is unknown whether WT1 is required for testis development after the initial steps of sex determination. To address this question, we generated a mouse strain carrying a Wt1 conditional knockout allele and ablated Wt1 function specifically in Sertoli cells by embryonic day 14.5, several days after testis determination. Wt1 knockout resulted in disruption of developing seminiferous tubules and subsequent progressive loss of Sertoli cells and germ cells such that postnatal mutant testes were almost completely devoid of these cell types and were severely hypoplastic. Thus, Wt1 is essential for the maintenance of Sertoli cells and seminiferous tubules in the developing testes. Of particular note, expression of the testis-determining gene Sox9 in mutant Sertoli cells was turned off at embryonic day 14.5 after Wt1 ablation, suggesting that WT1 regulates Sox9, either directly or indirectly, after Sry expression ceases. Our data, along with previous work demonstrating the role of Wt1 at early stages of gonadal development, thus indicate that Wt1 is essential at multiple steps in testicular development.
Keywords: Sertoli cell, testicular cord, Müllerian duct, Amh, Sox8
Mammalian testis determination is initiated by the expression of SRY. This Y-linked gene encodes a DNA-binding protein that is thought to up-regulate genes critical for the commitment of somatic cells of the genital ridge to become Sertoli cells. The direct targets of SRY remain elusive, although both in vivo and in vitro data suggest that one may be Sox9 (1–3), which itself plays a critical role in testis determination (4–9). Similarly, an important role for WT1 in genital tract development was recognized based on the observation of sex reversal or genital tract anomalies in XY patients with heterozygous germ-line WT1 mutations‖ (10) ranging from entire gene deletions to missense mutations. Variable expressivity, even among patients with a similar type of mutation, has been observed (11), suggesting a model whereby a critical threshold level of wild-type WT1 protein is required for normal testis determination and development, and stochastic variability in protein expression results in phenotypic variability.
In mice, Wt1 is required for the survival and proliferation of cells in the genital ridge. Although present at embryonic day (E) 10.5, the genital ridge fails to thicken in Wt1−/− animals, and no gonads are detectable by E14 (12). Commitment of the murine XY gonad to a testicular phenotype is first detectable at E12.5. Because of the complete gonadal agenesis exhibited by Wt1−/− mice and the observation of no testicular anomalies in Wt1+/− mice (12), assessing the role of Wt1 in testis determination and subsequent development has been difficult. To date, in vivo assessment of this role has come primarily from studies of mice expressing only one of the two Wt1 “kts” isoforms (13). These studies indicated that Wt1 plays a role in testis determination, perhaps by up-regulating Sry, but a further role for Wt1 in testicular development independent of Sry could not be assessed by this model. Consistent with the previous observation of apoptosis of the genital ridge in Wt1−/− animals, hypoplastic testes with dysgenic tubules containing no Wt1−/− Sertoli cells are observed in ≈40% of highly chimeric Wt1−/− ↔ XY adult males (14).
The continued expression of Wt1 in the mature testes suggests a role for Wt1 at later stages of testicular development, as have in vitro studies implicating Wt1 in the up-regulation of anti-Müllerian hormone, Amh, (also known as Müllerian inhibiting substance, Mis) in the differentiated testes (15). However, in vivo assessment of the possible role of Wt1 in the XY gonad after testis determination has been problematic because of apoptosis of the Wt1−/− genital ridge. To circumvent this problem, we generated a mouse strain (Wt1flox) carrying a Wt1 conditional knockout allele. Crossing it with the Wt1− strain (12) and the AMH-Cre transgenic strain, which expresses Cre in Sertoli cells soon after testis commitment (16), we generated Wt1−/flox; AMH-Cre males in which we could ablate Wt1 function in Sertoli cells and determine its role in the committed testes.
Results
Generation of Wt1 Conditional Knockout (Wt1flox) Mouse Strain.
By homologous recombination a Wt1 allele in which exons 8 and 9 are flanked by loxP sites (Fig. 1A–D) was introduced into the mouse germ line. These two exons encode the second and third of the four DNA-binding zinc-finger domains of Wt1. Missense and truncating mutations in exons 8 and 9 are observed in patients, and the critical functional importance of the two domains is further underscored by in vitro studies (15, 17, 18). Expression of Cre recombinase results in the in-frame deletion of exons 8 and 9 (Fig. 1E) and generates an allele (Wt1Δ) encoding a shortened Wt1 protein lacking zinc fingers 2 and 3. Germ-line generation of the Wt1Δ allele was achieved by using the oocyte-specific Zp3-cre transgene. Consistent with the expectation that the Wt1Δ allele acts as a null allele, Wt1Δ/Δ embryos from Wt1+/Δ × Wt1+/Δ crosses died during embryogenesis at the same developmental stage (E13–E15) as do Wt1−/− embryos. Additionally, Wt1+/Δ animals were viable and phenotypically normal, indicating that the Wt1Δ shortened protein does not act in a dominant negative manner (data not shown).
Fig. 1.
Generation of the Wt1flox mouse strain. (A) Scheme for generating animals carrying the conditional knockout allele (Wt1flox) and the recombined allele (Wt1Δ). (B) Southern blot of EcoRV restricted ES cell DNA probed with 5′ probe yielded a 7.14-kb fragment in recombinant animals in addition to the wild-type 5.34-kb fragment, confirming insertion of the targeting construct into the Wt1 locus. (C) PCR genotyping of wild-type and Wt1flox allele using primers loxpF and loxpR. (D) PCR genotyping of wild-type and Wt1flox allele using primers 1.75 and 1.55. (E) PCR detection of recombined Wt1Δ allele using primers ckodelF and 1.55.
Generation of Wt1−/flox; AMH-Cre Mice and Validation of Conditional Mutation System.
To achieve somatic ablation of Wt1 in testes, we introduced the Sertoli cell-expressed AMH-Cre transgene and the Wt1-null allele into the Wt1flox strain to obtain Wt1−/flox; AMH-Cre males. By immunohistochemistry (IHC), we detected Cre expression specifically in Sertoli cells at E14.5 but not at E13.5 (Fig. 2A). This finding is consistent with the spatio-temporal expression pattern previously reported for the AMH-Cre strain (16); expression of the human AMH promoter-driven transgene occurs 1–2 days later than the endogenous Amh gene. Cre-mediated excision of Wt1 exons 8 and 9 was detectable at E14.5 in testes, but not at E13.5 (Fig. 2B), consistent with the Cre expression pattern.
Fig. 2.
Expression of Cre-recombinase and recombination of Wt1flox allele. (A) IHC demonstrating expression of Cre-recombinase specifically in Sertoli cells of E14.5 Wt1−/flox; AMH-Cre testes (arrows) but not in mutant testes at E13.5 or in controls (Wt1−/flox). (B) Detection of recombined Wt1Δ allele specifically in testes of E14.5 Wt1−/flox; AMH-Cre embryos.
Viability and Gross Morphology of Wt1−/flox; AMH-Cre Males.
Wt1−/flox; AMH-Cre animals were fully viable. No gross abnormalities of external genitalia were observed in 7-week-old Wt1−/flox; AMH-Cre males (Fig. 3Aa), but testes size was only ≈10% of that of control littermates (Fig. 3Ab). The rest of the reproductive tract was also hypoplastic in the mutants (Fig. 3 Ac and Ad). Surprisingly, upon gross examination of Wt1−/flox; AMH-Cre males, they were found to have a uterus in addition to a vas deferens, epididymis, and seminal vesicles (Fig. 3Ad). Histologic analysis of the proximal portion of the reproductive tract from mutant males revealed structures with the coiled morphology and folded epithelium characteristic of oviduct (Fig. 7, which is published as supporting information on the PNAS web site). Thus, the normal regression of the Müllerian duct system was deficient in the Wt1−/flox; AMH-Cre males, whereas Wolffian duct differentiation was largely normal.
Fig. 3.
Phenotype of 7-week-old Wt1−/flox; AMH-Cre males. (A) Normal external genitalia of mutant male (Aa), severely reduced size of testes from three different Wt1−/flox; AMH-Cre males (Ab), and reproductive tracts from control male and Wt1−/flox; AMH-Cre male displaying reduced size and the development of both a vas deferens and uterus in mutant (Ac and Ad). (B) Testis sections stained with H&E or with anti-3β-HSD antibody and showing normal tubular architecture (asterisk) in control testes (Ba and Bb), lack of tubules in mutant testes (arrowhead) (Bd and Be), and presence of Leydig cells (lc) in both control (Bc) and Wt1−/flox; AMH-Cre (Bf) testes. Ep, epididymis; t, testis; vd, vas deferens; sv, seminal vesicle; u, uterus.
Aberrant Histology of Wt1−/flox; AMH-Cre Testes.
Histologically, Wt1−/flox; AMH-Cre testes from 7-week-old animals bore no resemblance to age-matched control testes. Mutant testes completely lacked the normal tubular architecture observed in control testes (Fig. 3 Ba and Bb) and consisted primarily of sheets of eosinophilic cells (Fig. 3 Bd and Be) which, by IHC analysis with anti-3β-HSD, were identified as Leydig cells (Fig. 3Bf). Normally, these cells are present in small numbers in the testicular interstitium (Fig. 3 Bc), but, in the Wt1−/flox; AMH-Cre testes, in the absence of tubules, they were observed in dense clusters interspersed with regions of fibroblast-like cells. Thus, Cre-mediated deletion of Wt1 at E14.5 in Sertoli cells had a profound effect on testis development, resulting in testes greatly reduced in size and composed almost entirely of cells normally found in the interstitium between tubules. Given the dramatically aberrant testicular histology we observed, Wt1−/flox; AMH-Cre animals are predicted to be infertile, although this has not rigorously been assessed.
Wt1 Deletion in Sertoli Cells Results in Testicular Cord Disruption.
To determine the time course of this aberrant development, we assessed testes from Wt1−/flox; AMH-Cre males between E13.5 and postnatal day 7 (P7), examining tissues from three to six animals at each time point. There was a high degree of concordance with respect to histology between mutant animals at the same age, and representative sections are presented. For controls, we assessed Wt1−/flox and Wt1+/flox; AMH-Cre and Wt1+/+ littermates at each time point. No difference was observed between these three control groups, and the control data presented are from mice of any of these three genotypes.
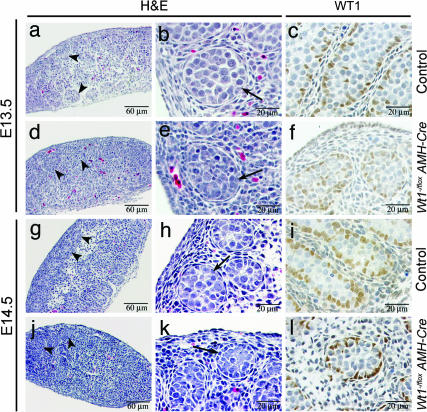
No aberrant pathology was discernable in any of the four Wt1−/flox; AMH-Cre animals at E13.5. Nascent development of sex cords could be seen at low magnification (arrowheads, Fig. 4d), and, at higher magnification, well organized tubule structures (Fig. 4e) were clearly present in Wt1−/flox; AMH-Cre testes. The polyclonal WT1 antibody, sc-192, is a WT1-specific rabbit antibody raised against a peptide corresponding to the 9 aa at the carboxyl terminus of WT1 (19). Therefore, it recognizes the mutant protein encoded by the Wt1Δ in-frame deletion, enabling us to employ this widely used marker of Sertoli cells to assess Sertoli cell location, number, and morphology in the Wt1−/flox; AMH-Cre testes. By IHC analyses, the location and morphology of Sertoli cells in Wt1−/flox; AMH-Cre testes (Fig. 4f) at E13.5 appeared normal (Fig. 4c), consistent with the lack of Cre-recombinase expression at this time point (Fig. 2A). At E14.5, when Cre-recombinase was expressed (Fig. 2A), Wt1−/flox; AMH-Cre testes appeared normal in all five embryos examined. Developing tubules were observed (arrowheads, Fig. 4j), and there was no salient alteration in architecture of the testes (Fig. 4 g and j), although Sertoli cell nuclei in mutant testes were less rounded than those in control testes (Fig. 4 i and l).
Fig. 4.
Mutant testes at E13.5 and E14.5. (d, e, j, and k) Normal testicular histology with normal tubules (arrowheads, arrows) observed in mutant testes at low and high magnification. (c, f, j, and l) WT1 immunostaining of Sertoli cells.
From E15.5 to P7, control testes exhibited progressive tubule development and organization with Leydig cells (lc) located between tubules (Fig. 5a–d). Over the same time period, Wt1−/flox; AMH-Cre testes increasingly lost normal tubular architecture (Fig. 5 i and j). At E15.5, only a few tubule-like structures were observed at low magnification in Wt1−/flox; AMH-Cre testes (asterisks, Fig. 5i). Most of the testes consisted of disorganized germ cells (gc) and Leydig cells (lc), which could be identified histologically (Fig. 5k). Apparent remnants of tubules consisted primarily of germ cells with few identifiable Sertoli cells (Fig. 5l). To identify positively Sertoli cells and germ cells, anti-WT1 and anti-GCNA-1 (germ cell nuclear antigen 1) antibodies (19, 20) were used for IHC. In control testes, GCNA-1-positive germ cells were localized in the center of tubules (Fig. 5f), surrounded by well organized WT1-positive Sertoli cells (Fig. 5e). However, in mutant testes, most of the Sertoli cells (Fig. 5o) and germ cells (Fig. 5s) were scattered and outside of any discernible tubular structure.
Fig. 5.
Disruption of seminiferous tubule architecture and loss of Sertoli cells and germ cells in Wt1−/flox; AMH-Cre testes between E15.5 and P7. (a–d) Progressive tubule development and organization in control testes from E15.5 to P7 shown by H&E staining. (e–h) Germ cells (GCNA-1-positive) and Sertoli cells (WT1-positive) located within tubules of control testes. (i and j) Progressive disruption of tubule architecture in Wt1−/flox; AMH-Cre testes (low magnification). (k and m) Predominant histology of E15.5 and P7 mutant testes (high magnification). (l and n) Histology of occasional aberrant tubules in E15.5 and P7 mutant testes (high magnification). (o–r) Sertoli cells in mutant testes identified by WT1 IHC. (s–v) Germ cells in mutant testes identified by GCNA-1 IHC. sc, Sertoli cells; gc, germ cells; lc, Leydig cell.
At P7, mutant testes consisted largely of fibroblast-like stromal cells and clusters of eosinophilic Leydig cells (Fig. 5 j and m), with only a few aberrant tubule-like structures present (asterisks, Fig. 5 j and n). No WT1-positive Sertoli cells were observed except in rare tubule remnants (Fig. 5 q and r). These data suggest that, after tubule disruption, mutant Sertoli cells eventually died or dedifferentiated into other cell types. Similarly, the few germ cells remaining were primarily in tubule remnants (Fig. 5 u and v).
Wt1 Ablation Results in Loss of SOX8, SOX9, and AMH Expression in Sertoli Cells.
Because the Sox8, Sox9, and Amh genes are expressed in Sertoli cells, we examined how their expression was affected by Wt1 inactivation. SOX9 and SOX8 proteins were detected in the Sertoli cells of both mutant and control testes at E13.5, and no difference was observed between three mutant males and three control animals assessed by IHC (Fig. 6a and b; d and e). Unexpectedly, unlike the robust expression in control testes, SOX9 and SOX8 expression was virtually absent in Wt1−/flox; AMH-Cre testes at E14.5 (Fig. 6 j and k), even though Sertoli cells could be identified in mutant testes both morphologically (arrows, Fig. 6 j and k) and by WT1 IHC (Fig. 4l). This result was consistently observed in the three mutant males assessed by IHC. These data demonstrate that SOX8 and SOX9 expression was down-regulated in mutant Sertoli cells and suggest that WT1 is required for the continued expression of these genes. As expected, given the reported role of both SOX9 and WT1 in regulating Amh transcription (21, 22), AMH expression, normal at E13.5, was also absent in mutant testes by E14.5 (Fig. 6l).
Fig. 6.
Loss of SOX9, SOX8, and AMH expression by E14.5 in Wt1−/flox; AMH-Cre Sertoli cells. Control testes show normal SOX9 (a and g), SOX8 (b and h), and AMH (c and i) expression in Sertoli cells at E13.5 and E14.5. SOX9 (d), SOX8 (e), and AMH (f) expression was normal in E13.5 Wt1−/flox; AMH-Cre testes but was virtually absent in E14.5 testes, even in those Sertoli cells associated with tubules (asterisks, j–l). sc, Sertoli cells.
Discussion
Because Wt1 is required for survival of the genital ridge, in vivo examination of its potential role at later stages of gonad development has been problematic. With the ability to somatically ablate Wt1 using our newly generated Wt1flox strain, we have now been able to assess the role of Wt1 after commitment of the indifferent gonad. The current study now provides conclusive in vivo evidence that Wt1 is essential, even after testis determination, for normal testis structure and function.
Wt1 Is Required for Maintenance of Testicular Architecture.
Although mutant and control testes were indistinguishable morphologically at E13.5 before expression of the AMH-Cre transgene, after Wt1 ablation, a dramatic and progressive loss of seminiferous tubular architecture and Sertoli cells and germ cells was observed beginning at E15.5. By P7, mutant testes were composed of Leydig cells and fibroblast-like cells, with only the occasional aberrant tubule remnant containing a few GCNA1- and WT1-positive cells. We speculate that these rare aberrant tubules are those in which Cre-mediated Wt1 inactivation did not occur in a large enough proportion of Sertoli cells within a tubule to trigger its complete disruption.
Given our observation that initially well organized tubules in mutant testes progressively lose their normal structure after Cre-mediated Wt1 ablation, we speculate that at least one result of Wt1 mutation is the dysregulation of genes that are critical for normal Sertoli cell–cell contacts, for Sertoli cell–basal lamina contacts, or for normal composition of the basal lamina, the components of which are synthesized by both Sertoli cells and the peritubular myoid cells (reviewed in ref. 23). Altered expression of these genes could be either a direct or indirect effect of losing the transcriptional regulatory function of WT1. Alternatively, the gene expression dysregulation resulting from Wt1 mutation may have set into motion a cellular response that secondarily affected the ability of Sertoli cells to maintain appropriate cell–cell and cell–matrix contacts.
We detected no increased apoptosis in mutant Sertoli cells (data not shown), suggesting that tubule disruption is not a result of Sertoli programmed cell death. We did, however, observe a dramatic decline in the number of Sertoli cells positive for the mitotic cell marker H3P beginning at E14.5 until, by E18.5, no H3P-positive Sertoli cells were observed (Fig. 8, which is published as supporting information on the PNAS web site). This cessation of Sertoli cell proliferation may be a result of altered cellular gene expression because of Wt1 ablation or secondarily triggered by the loss of normal cellular milieu because of tubule disaggregation. Given the known importance of cell–cell and cell–matrix interactions for Sertoli cell function, we favor this latter scenario but cannot exclude the former.
Loss of Germ Cells After Tubule Disruption in Mutant Testes.
GCNA1 is a germ cell-specific marker (20). We observed no salient difference in the number of GCNA1-positive cells in mutant and control testes at E15.5, but, as tubule structure disintegrated, there was a progressive loss of germ cells. This loss paralleled the loss of identifiable Sertoli cells and is consistent with the known interdependence of Sertoli cells and germ cells. Wt1 expression has been reported in E12 primordial germ cells (14). However, two lines of evidence suggest that it is unlikely that this expression played a role in the loss of germ cells in the Wt1−/flox; AMH-Cre testis. First, by IHC, we did not observe WT1-positive germ cells at E13.5 or later. Second, given the Sertoli cell-specific expression of Cre, we would not expect Wt1 ablation in germ cells; they would not be expected to display a phenotype. Therefore, we conclude that the loss of germ cells in mutant testes is secondary to Sertoli cell loss and/or the loss of tubular architecture.
No Apparent Loss of Leydig Cells in Mutant Testes.
IHC analysis using the Leydig cell marker, 3β-HSD, demonstrated an abundance of Leydig cells in embryonic mutant testes (data not shown), consistent with the predominant population of Leydig cells in adult mutant testes. We hypothesize that the high proportion of Leydig cells in mutant testes is a function of the absence of germ cells and Sertoli cells; with their loss, the testes have effectively collapsed, containing only the normal complement of Leydig and other interstitial cells.
Paracrine factors secreted by Sertoli cells are thought to be important for the development and function of Leydig cells (reviewed in ref. 24). Of particular note, testosterone production in mature Leydig cells in vitro has been reported to be enhanced by coculture with Sertoli cells (25). Therefore, our observations of continued proliferation of Leydig cells in the mutant testes and of serum testosterone levels within normal ranges in mutant animals (data not presented) were unexpected because they indicated that Leydig cells in the mutant testes were viable and functional even in the absence of Sertoli cells. These data suggest that Leydig cells at later stages of testes development (e.g., approximately E14.5 and onward) do not require Sertoli cells for proliferation and synthesis of testosterone. Thus, unlike Sertoli cells and germ cells, which in the absence of seminiferous tubules and appropriate cell–cell interactions are unable to survive, Leydig cells, in their normal extratubular location, can survive and function.
Loss of SOX9 Expression in Wt1−/flox; AMH-Cre testes.
Unexpectedly, expression of SOX9 protein in the mutant testes was virtually silenced at E14.5 even though Sertoli cells were clearly present in morphologically intact tubules. Sox9 is normally expressed at low levels in the genital ridge of both XX and XY animals, and its expression is up-regulated in the male gonad at approximately E11.0, soon after Sry expression is detectable (2). Sry is down-regulated at approximately E12.5, but Sox9 expression continues in the Sertoli cells of the committed testis (1, 2, 26). These data suggest that Sox9 is up-regulated, directly or indirectly, by SRY, but that its continued expression is independent of SRY.
Gonads from mice expressing only the “−kts” isoform of Wt1 display reduced Sry expression and complete sex reversal, suggesting that WT1 activates Sry (14). The loss of SOX9 expression in Wt1−/flox; AMH-Cre testes, however, occurred approximately 2 days after Sry is normally down-regulated. Thus, we conclude that the lack of SOX9 expression at E14.5 is independent of WT1's putative function in up-regulating Sry and that WT1 is required for the continued expression of Sox9 in the committed testis. The identification of an evolutionarily conserved region 5′ of Sox9 that contains a putative WT1-binding site supports the notion that WT1 is directly involved in the regulation of Sox9 expression (27), but future studies will be required to demonstrate a direct role of WT1 in Sox9 regulation. Regardless of whether it is a direct or indirect effect, our data provide an indication that WT1 is required for the continued expression of Sox9.
Loss of AMH Expression and Development of Müllerian Ducts in Mutant Males.
Müllerian duct regression in XY animals is initiated by AMH and proceeds in a cranial to caudal manner. The Amh gene is normally expressed beginning at approximately E11.5 in the mouse (28), but the Müllerian duct is sensitive to its effects only during a narrow window of development (E13.5–E14.5) during which apoptosis of the ductal epithelium, morphologic changes in periductal mesenchyme, and sexually dimorphic expression of Lhx1, a gene required for Müllerian duct differentiation, begin to be observed (29–31). Although normal at E13.5, Sertoli cell expression of AMH expression in mutant males was negligible at E14.5. The presence of uteri and, more proximally, coiled structures with morphological and histological features of oviducts in mutant males indicates a failure of both cranial (oviducts) and more caudal (uterus) regression of the Müllerian duct. These data suggest that the window of time during which AMH can effect Müllerian duct regression is narrower than previously thought.
In vitro and in vivo studies have identified a functional SOX9-binding site in the proximal promoter of Amh that is required for Amh expression. Steroidogenic factor 1 (NR5A1, also known as SF1) is thought to enhance this interaction, and NR5A1/SF1 functional binding sites have been identified within the Amh promoter (22, 32, 33). In vitro studies have suggested that WT1 synergizes with NR5A1/SF1 to activate the Amh promoter, but the mechanism for this activation is not clear. WT1 has also been reported to positively regulate the Nr5a1/Sf1 gene (34), but NR5A1/SF1 expression was not noticeably reduced in the three mutant E15.5 testes we assessed by IHC (Fig. 9, which is published as supporting information on the PNAS web site), suggesting that WT1 is not critical for NR5A1/SF1 expression in Sertoli cells at this stage of development. Interestingly, missense mutations in Wt1 exon 9, one of the exons deleted from the Wt1Δ allele, abolish the ability of WT1 to activate Amh expression (15). Therefore, the lack of AMH expression we observed in mutant testes may be due to loss of SOX9 expression, loss of WT1 function, or both.
Somatic Mutation of Wt1 in the Committed Testis Results in a More Severe Phenotype than Somatic Mutation of Sox9.
Our data suggest a model whereby Wt1 ablation between E13.5 and E14.5 results in the loss of SOX9 expression, which in turn results in loss of AMH expression and lack of Müllerian duct regression. However, interestingly, the phenotype of Wt1−/flox; AMH-Cre males was considerably more profoundly aberrant than that observed in Sox9flox/flox; Sf1-Cre males in which Sox9 itself was ablated in the male gonad (7), suggesting that WT1 has an additional critical function(s) in maintaining normal testicular structure/function that is independent of its role in Sox9 regulation.
Another Sox Group E member, SOX8, is expressed in testes and has also been implicated in the regulation of Amh (35, 36), and analyses of single and compound Sox8 and Sox9 mutant mice have suggested a coordinated expression and possible functional redundancy of SOX8 and SOX9 in the early XY gonad (7). We observed extinguished SOX8 expression at E14.5 in mutant testes, but whether this was due to loss of SOX9 expression or a direct or indirect result of Wt1 mutation is not known. The Wt1−/flox; AMH-Cre testes phenotype was considerably more pronounced and penetrant than that of Sox9flox/flox; Sox8−/+; Sf1-Cre males in which testes were observed, further suggesting that Wt1 has a critical role in maintaining testes structure and function independent of Sox9 and Sox8. Analysis of Sox9flox/flox; Sox8−/+ males carrying the same robustly expressing AMH-Cre transgene as used in the studies presented here will be required to address this further.
In summary, our work expands the role of Wt1 in testicular development and demonstrates that WT1 is required for SOX9 expression in the testis, independently of SRY, and also that it is essential for the maintenance of Sertoli cells and seminiferous tubules in the developing testis. Thus, WT1 plays essential roles at multiple steps in testis development in the mouse and, likely, given its high degree of sequence conservation (37), in a wide range of vertebrates.
Materials and Methods
Generation of a Wt1 Conditional Knockout Mouse Strain.
All animal work was carried out in accordance with institutional animal care and use committee (IACUC) regulations. A targeting vector in which Wt1 exons 8 and 9 were flanked by a loxP site and a loxP-neo-loxP cassette (Fig. 1A) was constructed and introduced into mouse embryonic stem cells (AB1, 129/SvEv) by electroporation. Resultant G418-resistant clones were genotyped by Southern blotting; introduction of the targeting construct into the Wt1 locus by means of homologous recombination resulted in the generation of a 7.14-kb EcoRV fragment (Fig. 1B) detected with the 5′ probe indicated in Fig. 1A. Exons 7, 8, 9, and 10 and flanking intronic sequence were amplified from several clones, and PCR products were sequenced to confirm that intact loxP sites had been introduced into the locus and that no other sequence alterations had occurred. Clones were then injected into C57BL/6 (B6) blastocysts. Resultant male chimeras were identified by coat color and mated with wild-type females. Tail biopsies of agouti-pigmented F1 animals were genotyped by using a primer set specific to the neo cassette. Animals carrying the flox-neo allele were then mated with CMV-Cre animals (22). Progeny in which the neo cassette had been excised by means of Cre-mediated recombination were identified by using primers (1.75 and 1.55) flanking the 3′ loxP site (Fig. 1A).
Generation of Wt1−/flox; AMH-Cre Mice.
Wt1+/flox mice were mated with mice carrying the Wt1-null allele (Wt1+/−) (12) and the AMH-Cre transgene mice (16).
Genotyping.
DNA isolated from tail biopsies (postnatal) or from limbs (embryos) was used for genotyping. The presence of the Wt1flox allele was determined by PCR amplification using the loxpF/loxpR (Fig. 1C) and 1.75/1.55 (Fig. 1D) primer sets. The recombined Wt1flox allele (Wt1Δ) was detected by PCR amplification using the ckodelF and 1.55 primers (Fig. 1E). Genotyping for the Wt1-null allele and the AMH-Cre transgene was carried out as described (12, 16).
Tissue Collection and Histologic Analysis.
Testes and reproductive tracts were dissected immediately after euthanasia. Testes were dissected from four mutant male embryos at E13.5, five at E14.5, five at E15.5, and six at E16.5. Tissues were collected from three mutant males at each E17.5, E18.5, and P7 time point and from five 7-week-old mutant males. Tissues from three to four control littermates were also collected at each time point. Tissues were fixed in 4% paraformaldehyde for up to 24 h, stored in 70% ethanol, and embedded in paraffin. Four-micrometer-thick sections were cut and mounted on glass slides. After deparaffinization, slides were stained with H&E for histologic analyses. Slides were examined from all mutant embryos/animals collected.
Immunohistochemical Analysis.
IHC analysis of tissues from at least three mutant males at each time point was performed by using a Vectastain ABC (avidin–biotin–peroxidase) kit (Vector Laboratories, Burlingame, CA) as recommended. Antibodies to WT1 (sc-192) and AMH (sc-6886) were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Anti-SOX9 antibody (AB5535) was purchased from Chemicon (Temecula, CA), and antibody to CRE (69050) was obtained from Novagen (La Jolla, CA). The anti-PCNA-1 antibody was generously given to us by G. Enders (University of Kansas Medical Center, Kansas City, KS) (20); the anti-3β-HSD antibody was a kind gift from C. Richard Parker (University of Alabama at Birmingham) (38), and the anti-SOX8 antibody was generously provided by M. Wegner (University of Erlangen, Erlangen, Germany) (39). The IHC procedure was as described (40). Stained slides were examined with a Leica DMR Epifluorescence Microscope, and images were captured by a Hamamatsu CCD camera.
Supplementary Material
Acknowledgments
We thank Allan Bradley (Wellcome Trust Sanger Institute, Cambridge, U.K.) for ABI ES and SNL 76/7 STO cells; George Enders, Richard Parker, and Dr. Michael Wegner for antibodies; and Marvin Meistrich for helpful discussions. This work was supported by National Institutes of Health (NIH) Grants CA78257, CA34936, and HD30284. Blastocyst injection, veterinary resources, and DNA sequencing were partially supported by NIH Cancer Center Support (Core) Grant CA16672. This work was also supported in part by the Odyssey Program and the Theodore N. Law Award for Scientific Achievement at M. D. Anderson Cancer Center.
Abbreviations
- IHC
immunohistochemistry
- En
embryonic day n
- Amh
anti-Müllerian hormone
- Pn
postnatal day n
- GCNA-1
germ cell nuclear antigen 1.
Note Added in Proof.
Rao et al (41) have observed reduced fertility accompanied by loss of germ cells and aberrant Sertoli cell morphology when Wt1 is down-regulated postnatally.
Footnotes
Conflict of interest statement: No conflicts declared.
This paper was submitted directly (Track II) to the PNAS office. J.K. is a guest editor invited by the Editorial Board.
Huff, V., Villalba, F., Strong, L. C. & Saunder, G. F. (1991) Am. J. Hum. Genet. 49, 44 (Abstr.).
References
- 1.Kent J., Wheatley S. C., Andrews J. E., Sinclair A. H., Koopman P. Development (Cambridge, U.K.) 1996;122:2813–2822. doi: 10.1242/dev.122.9.2813. [DOI] [PubMed] [Google Scholar]
- 2.Morais da Silva S., Hacker A., Harley V., Goodfellow P., Swain A., Lovell-Badge R. Nat. Genet. 1996;14:62–68. doi: 10.1038/ng0996-62. [DOI] [PubMed] [Google Scholar]
- 3.Sekido R., Bar I., Narvaez V., Penny G., Lovell-Badge R. Dev. Biol. 2004;274:271–279. doi: 10.1016/j.ydbio.2004.07.011. [DOI] [PubMed] [Google Scholar]
- 4.Wagner T., Wirth J., Meyer J., Zabel B., Held M., Zimmer J., Pasantes J., Bricarelli F. D., Keutel J., Hustert E., et al. Cell. 1994;79:1111–1120. doi: 10.1016/0092-8674(94)90041-8. [DOI] [PubMed] [Google Scholar]
- 5.Foster J. W., Dominguez-Steglich M. A., Guioli S., Kowk G., Weller P. A., Stevanovic M., Weissenbach J., Mansour S., Young I. D., Goodfellow P. N., et al. Nature. 1994;372:525–530. doi: 10.1038/372525a0. [DOI] [PubMed] [Google Scholar]
- 6.Bishop C. E., Whitworth D. J., Qin Y., Agoulnik A. I., Agoulnik I. U., Harrison W. R., Behringer R. R., Overbeek P. A. Nat. Genet. 2000;26:490–494. doi: 10.1038/82652. [DOI] [PubMed] [Google Scholar]
- 7.Chaboissier M. C., Kobayashi A., Vidal V. I., Lutzkendorf S., van de Kant H. J., Wegner M., de Rooij D. G., Behringer R. R., Schedl A. Development (Cambridge, U.K.) 2004;131:1891–1901. doi: 10.1242/dev.01087. [DOI] [PubMed] [Google Scholar]
- 8.Barrionuevo F., Bagheri-Fam S., Klattig J., Kist R., Taketo M. M., Englert C., Scherer G. Biol. Reprod. 2006;74:195–201. doi: 10.1095/biolreprod.105.045930. [DOI] [PubMed] [Google Scholar]
- 9.Vidal V. P., Chaboissier M. C., de Rooij D. G., Schedl A. Nat. Genet. 2001;28:216–217. doi: 10.1038/90046. [DOI] [PubMed] [Google Scholar]
- 10.Pelletier J., Bruening W., Kashtan C. E., Mauer S. M., Manivel J. C., Striegel J. E., Houghton D. C., Junien C., Habib R., Fouser L., et al. Cell. 1991;67:437–447. doi: 10.1016/0092-8674(91)90194-4. [DOI] [PubMed] [Google Scholar]
- 11.Schumacher V., Scharer K., Wuhl E., Altrogge H., Bonzel K.-E., Guschmann M., Neuhaus T. J., Pollastro R. M., Kuwertz-Broking E., Bulla M., et al. Kidney Int. 1998;53:1594–1600. doi: 10.1046/j.1523-1755.1998.00948.x. [DOI] [PubMed] [Google Scholar]
- 12.Kreidberg J. A., Sariola H., Loring J. M., Maeda M., Pelletier J., Housman D., Jaenisch R. Cell. 1993;74:679–691. doi: 10.1016/0092-8674(93)90515-r. [DOI] [PubMed] [Google Scholar]
- 13.Hammes A., Guo J. K., Lutsch G., Leheste J. R., Landrock D., Ziegler U., Gubler M. C., Schedl A. Cell. 2001;106:319–329. doi: 10.1016/s0092-8674(01)00453-6. [DOI] [PubMed] [Google Scholar]
- 14.Natoli T. A., Alberta J. A., Bortvin A., Taglienti M. E., Menke D. B., Loring J., Jaenisch R., Page D. C., Housman D. E., Kreidberg J. A. Dev. Biol. 2004;268:429–440. doi: 10.1016/j.ydbio.2003.12.033. [DOI] [PubMed] [Google Scholar]
- 15.Nachtigal M. W., Hirokawa Y., Enyeart-VanHouten D. L., Flanagan J. N., Hammer G. D., Ingraham H. A. Cell. 1998;93:445–454. doi: 10.1016/s0092-8674(00)81172-1. [DOI] [PubMed] [Google Scholar]
- 16.Lecureuil C., Fontaine I., Crepieux P., Guillou F. Genesis. 2002;33:114–118. doi: 10.1002/gene.10100. [DOI] [PubMed] [Google Scholar]
- 17.Royer-Pokora B., Beier M., Henzler M., Alam R., Schumacher V., Weirich A., Huff V. Am. J. Med. Genet. A. 2004;127:249–257. doi: 10.1002/ajmg.a.30015. [DOI] [PubMed] [Google Scholar]
- 18.Hossain A., Saunders G. F. J. Biol. Chem. 2001;276:16817–16823. doi: 10.1074/jbc.M009056200. [DOI] [PubMed] [Google Scholar]
- 19.Silberstein G. B., Van Horn K., Strickland P., Roberts C. T., Jr., Daniel C. W. Proc. Natl. Acad. Sci. USA. 1997;94:8132–8137. doi: 10.1073/pnas.94.15.8132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Enders G. C., May J. J., II Dev. Biol. 1994;163:331–340. doi: 10.1006/dbio.1994.1152. [DOI] [PubMed] [Google Scholar]
- 21.De Santa Barbara P., Bonneaud N., Boizet B., Desclozeaux M., Moniot B., Sudbeck P., Scherer G., Poulat F., Berta P. Mol. Cell. Biol. 1998;18:6653–6665. doi: 10.1128/mcb.18.11.6653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Arango N. A., Lovell-Badge R., Behringer R. R. Cell. 1999;99:409–419. doi: 10.1016/s0092-8674(00)81527-5. [DOI] [PubMed] [Google Scholar]
- 23.Skinner M. K. In: Sertoli Cell Biology. Skinner M. K., Griswold M. D., editors. New York: Elsevier; 2005. pp. 317–328. [Google Scholar]
- 24.Skinner M. K. Endocr. Rev. 1991;12:45–77. doi: 10.1210/edrv-12-1-45. [DOI] [PubMed] [Google Scholar]
- 25.Lejeune H., Sanchez P., Saez J. M. Int. J. Androl. 1998;21:129–140. doi: 10.1046/j.1365-2605.1998.00105.x. [DOI] [PubMed] [Google Scholar]
- 26.Wilhelm D., Martinson F., Bradford S., Wilson M. J., Combes A. N., Beverdam A., Bowles J., Mizusaki H., Koopman P. Dev. Biol. 2005;287:111–124. doi: 10.1016/j.ydbio.2005.08.039. [DOI] [PubMed] [Google Scholar]
- 27.Bagheri-Fam S., Ferraz C., Demaille J., Scherer G., Pfeifer D. Genomics. 2001;78:73–82. doi: 10.1006/geno.2001.6648. [DOI] [PubMed] [Google Scholar]
- 28.Munsterberg A., Lovell-Badge R. Development (Cambridge, U.K.) 1991;113:613–624. doi: 10.1242/dev.113.2.613. [DOI] [PubMed] [Google Scholar]
- 29.Dyche W. J. J. Morphol. 1979;162:175–209. doi: 10.1002/jmor.1051620203. [DOI] [PubMed] [Google Scholar]
- 30.Allard S., Adin P., Gouedard L., di Clemente N., Joss N., Orgebin-Crist M.-C., Picard J.-Y., Xavier F. Development (Cambridge, U.K.) 2000;127:3349–3360. doi: 10.1242/dev.127.15.3349. [DOI] [PubMed] [Google Scholar]
- 31.Kobayashi A., Shawlot W., Kania A., Behringer R. R. Development (Cambridge, U.K.) 2004;131:539–549. doi: 10.1242/dev.00951. [DOI] [PubMed] [Google Scholar]
- 32.Shen W.-H., Moore C. C., Ikeda Y., Parker K. L., Ingraham H. A. Cell. 1994;77:651–661. doi: 10.1016/0092-8674(94)90050-7. [DOI] [PubMed] [Google Scholar]
- 33.Watanabe K., Clarke T. R., Lane A. H., Wang X., Donahoe P. K. Proc. Natl. Acad. Sci. USA. 2000;97:1624–1629. doi: 10.1073/pnas.97.4.1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wilhelm D., Englert C. Genes Dev. 2002;16:1839–1851. doi: 10.1101/gad.220102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schepers G. E., Bullejos M., Hosking B. M., Koopman P. Nucleic Acids Res. 2000;28:1473–1480. doi: 10.1093/nar/28.6.1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schepers G., Wilson M., Wilhelm D., Koopman P. J. Biol. Chem. 2003;278:28101–28108. doi: 10.1074/jbc.M304067200. [DOI] [PubMed] [Google Scholar]
- 37.Kent J., Coriat A. M., Sharpe P. T., Hastie N. D., van Heyningen V. Oncogene. 1995;11:1781–1792. [PubMed] [Google Scholar]
- 38.Parker C. R., Jr., Faye-Petersen O., Stankovic A. K., Mason J. I., Grizzle W. E. Endocr. Res. 1995;21:69–80. doi: 10.3109/07435809509030422. [DOI] [PubMed] [Google Scholar]
- 39.Stolt C. C., Schmitt S., Lommes P., Sock E., Wegner M. Dev. Biol. 2005;281:309–317. doi: 10.1016/j.ydbio.2005.03.010. [DOI] [PubMed] [Google Scholar]
- 40.Gao F., Maiti S., Sun G., Ordonez N. G., Udtha M., Deng J. M., Behringer R. R., Huff V. Mol. Cell. Biol. 2004;24:9899–9910. doi: 10.1128/MCB.24.22.9899-9910.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rao M. K., Pham J., Imam J. S., MacLean J. A., Murali D., Furuta Y., Sinha-Hikim A. P., Wilkinson M. F. Gene Dev. 2006;20:147–152. doi: 10.1101/gad1367806. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.