Abstract
In many animal societies, dominant individuals monopolize reproduction, but the tactics they employ to achieve this are poorly understood. One possibility is that aggressive dominants render their subordinates infertile by inducing chronic physiological “stress.” However, this hypothesis has been discarded largely for cooperatively breeding species, where reproductive monopolies are often extreme. Here we provide strong support for the stress-related suppression hypothesis in a cooperative mammal, the meerkat (Suricata suricatta). When pregnant, dominant females subject some subordinate females to escalating aggression, culminating in temporary evictions from the group. While evicted, subordinate females suffer chronic elevation of their glucocorticoid adrenal hormone levels, reproductive down-regulation (reduced pituitary sensitivity to gonadotropin-releasing hormone), reduced conception rates, and increased abortion rates. Rather than constantly harassing all subordinate females, dominants only become aggressive when pregnant themselves (when subordinate reproduction would otherwise conflict with their own) and target those females with whom reproductive conflict is most likely (older, pregnant, and more distantly related females). Our findings suggest that dominant female meerkats employ stressful evictions to suppress reproduction among their probable competitors, when attempting to breed themselves. Given the lack of evidence for stress-related suppression in other cooperative breeders to date, it is clear that social stress alone cannot account for the reproductive failure of subordinates across such societies. However, our findings raise the possibility that, in some cooperative breeders at least, dominants may employ stress-related suppression as a backup mechanism to guard against lapses in reproductive restraint by their subordinates.
Keywords: cooperative breeding, dominant control, physiological, reproductive restraint, reproductive skew
In many animal societies, dominant individuals monopolize reproduction, but the tactics they employ to achieve this remain poorly understood (1). One possibility is that dominants render their subordinates infertile by inducing chronic stress [resulting in continual activation of the hypothalamic-pituitary-adrenal (HPA) axis and chronic elevation of glucocorticoid (GC) adrenal hormone levels] through frequent attacks (2–4). This hypothesis is attractive because chronic stress is known to compromise fertility in a variety of taxa (5), and some studies of social vertebrates have supported the prediction that subordinates, who are commonly the target of aggression, should show elevated GC levels (reviewed in refs. 6 and 7). However, recent studies focusing on cooperatively breeding societies in particular (where reproductive monopolies are at their most extreme) have revealed that subordinate group members commonly show GC levels equal to or actually lower than those of dominants (reviewed in ref. 7), and experimental work on two cooperative species found no evidence of a role for elevated GCs in subordinate infertility (8, 9). These findings, coupled with the low frequency of overt aggression in many cooperative species, have led to the suggestion that reproductive monopolies in cooperatively breeding societies are not maintained through social stress (7, 8).
However, it may be premature to reject a role for stress-related suppression in all cooperatively breeding species. It is widely recognized that subordinates in cooperative societies commonly show a degree of reproductive restraint due to factors such as a lack of access to unrelated breeding partners, poor body condition, or underdeveloped foraging skills that reduce their expected payoff from attempted reproduction (10–12). Under these circumstances, dominants would only benefit from directing stress-related suppression at the subset of subordinates who would otherwise attempt to breed. Furthermore, dominants may only benefit from attacking these likely breeders at specific times, e.g., during seasonal mating periods or at times when the dominant is rearing her own young (when any young born to subordinates would otherwise compete with those of the dominant; ref. 13). If stress-related suppression was used in this way (directed at only a subset of subordinates at critical times to guard against lapses in restraint), it could be a difficult phenomenon to detect.
Here we test the stress-related suppression hypothesis in the cooperatively breeding meerkat (Suricata suricatta) by using an approach that should maximize our chances of detecting any role it may play: We investigate specifically how periods of dominant aggression, when they do occur, affect the adrenal and reproductive physiology of the targeted subordinates. Meerkats are small (<1 kg) social mongooses that live in cooperatively breeding groups of up to 50 individuals in the arid regions of southern Africa. A single female in each group is behaviorally dominant to, and typically older and heavier than, all other females (13, 14). This “dominant female” largely monopolizes reproduction within the group, conceiving at substantially higher rates than subordinates and producing >80% of the pups that survive their first month of life (13). Although the low conception rates of subordinate females (and their associated low estrogen levels) can sometimes be attributed to a lack of access to unrelated breeding partners within their groups (11, 13), subordinate females still conceive at lower rates than dominants even when they have access to unrelated males (11), suggesting that other processes regulate their fertility as well. Aggression is uncommon outside breeding periods, but ≈1 month before the dominant female gives birth, she subjects some subordinate females to escalating attacks, commonly driving them from the group until her own litter is born (14). During these temporary evictions, subordinate females are unable to forage or sleep with the main group, spend their time foraging either alone or in small parties with other evictees elsewhere on the group’s territory, and are repeatedly chased and attacked by the dominant female (sometimes other group members join these attacks) whenever they encounter the group (14). Once the dominant female has given birth, the evictees are allowed to return and help to rear the dominant’s litter (14). Although previous work has interpreted these temporary evictions as a counterinfanticide strategy (14), it is also possible that this period of sustained aggression toward subordinate females serves to compromise their fertility when the dominant is attempting to breed by inducing chronic stress responses. We combine nine years of life history monitoring with detailed behavioral and physiological data to investigate this possibility.
Specifically, we test four predictions arising from the stress-related suppression hypothesis. Relative to subordinate females within their groups, evicted females should show: (i) elevated GC levels (we monitor GC metabolite excretion in fecal samples); (ii) down-regulation of their reproductive physiology [we monitor circulating luteinising hormone, estradiol, progesterone, and prolactin and assess pituitary sensitivity to an exogenous gonadotropin-releasing hormone (GnRH) challenge]; (iii) reduced conception rates and/or increased abortion rates. We also monitor changes in subordinate female body weight while evicted from their groups, because loss of body condition also could contribute to any reproductive down-regulation detected. Finally, we investigate whether (iv) dominant females evict those subordinate females with whom reproductive conflict is most likely: those who are most likely to become pregnant (older females and those with access to unrelated breeding partners; ref. 13) and those who already are pregnant.
Results
Females <9 months of age rarely conceived (2.9%, 4 of 134 females, which rose to 20.1% of females by 12 months of age), and were not subjected to temporary evictions. Subordinate females >9 months of age were evicted temporarily from their groups by the pregnant dominant on 225 of 527 (42.7%) possible occasions (instances when a subordinate of this age was a group member when the dominant conceived). Subordinate females were evicted a median of 20 days [interquartile range (IQR) 11–29 days] before the birth of the dominant’s litter (gestation length was 70 days), and only returned to the group once the litter had been born (14). Evictees typically remained on their group’s territory throughout this period and were seen encountering their groups (one or more group members clearly reacting to their presence) on a median of 35.5% of days spent evicted (IQR 16.7–60.0%). On at least 58% of these encounter days, evictees were seen suffering repeated attacks and chases by the dominant female [at a median rate of 0.66 events per encounter day observation hour (IQR 0.36–1.48), ranging up to 10 events per hour]. These rates are likely to be substantial underestimates because encounters, attacks, and chases have been included only if the subject could be confidently identified as an evicted female (foreign individuals are also frequently encountered, attacked, and chased). Evictees commonly suffered injuries to the tail-base region and hind-quarters, where the dominant focused her attacks. Returning evictees joined their groups a median of 3 days (IQR 1–8 days) after the birth of the dominant’s litter.
Do Evicted Females Show Elevated GC Levels?
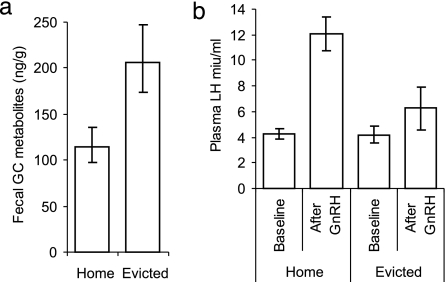
Subordinate females showed substantially higher levels of GC metabolites in their fecal samples while evicted than when within their groups [Fig. 1a; General(ized) Linear Mixed Model (GLMM): χ21 = 16.33, P < 0.001; based on 188 fecal GC measures from 58 subordinate females from 10 groups] while controlling for variation due to time of day (χ21 = 9.45, P = 0.002). Within-female paired comparisons of mean fecal GC metabolite levels between the evicted and within-group contexts corroborate this finding (Wilcoxon signed-ranks: W = 101.0, n = 18 females, P = 0.027). None of the other variables tested in our multivariate analysis were significantly associated with GC metabolite levels.
Fig. 1.
Effect of eviction on subordinate female adrenal and reproductive physiology. (a) Evicted females show significantly elevated levels of GC metabolites in their fecal samples. (b) Evicted females experience down-regulation of their pituitary responsiveness to an exogenous GnRH challenge. Bars present means ± 1 SE. (a shows predicted means after controlling for significant diel variation.)
Do Evicted Females Show Down-Regulated Reproductive Physiology?
Evicted females exhibited substantial down-regulation of their pituitary sensitivity to an exogenous GnRH challenge (Fig. 1b; the GnRH-induced increases in plasma luteinizing hormone (LH) levels were significantly smaller in evictees than in subordinate females within their groups: general(ized) linear model interaction term F1,50 = 6.21, P = 0.016). Plasma LH levels increased significantly in response to a GnRH challenge when subordinate females were in their groups (Fig. 1b, home; paired t test: t = 6.42, n = 18 females, P < 0.001), but showed no significant response to GnRH challenge, while subordinate females were evicted (Fig. 1b, evicted; paired t test: t = 1.25, n = 9 females, P = 0.25). This difference arose not because baseline LH levels differed between the two contexts (Fig. 1b; unpaired t test: t = 0.064, n = 18 and 9, P = 0.95), but rather because evicted females showed significantly lower postchallenge LH levels than females in their groups (Fig. 1b; unpaired t test: t = 2.68, n = 18 and 9 females, P = 0.013). Compared with subordinate females in their groups, evicted females did not show significantly different plasma levels of estradiol [Mann–Whitney: t = 155.0, n = 13 and 12 females, P = 0.98; medians (IQR): within-group = 0.00 (0.00–0.00) pg/ml, evicted = 0.00 (0.00–0.00) pg/ml], progesterone [Mann–Whitney: t = 82.5, n = 9 and 9 females, P = 0.83; medians (IQR): within-group = 0.60 (0.0–0.73) ng/ml, evicted = 0.60 (0.0–1.1) ng/ml], or prolactin [unpaired t test: t = 0.32, n = 16 and 11 females, P = 0.75; means ± SE: within-group = 4.98 ± 0.51 ng/ml, evicted = 5.19 ± 0.33 ng/ml]. Because most of the samples in the estradiol analysis (21 of 25) had undetectable levels of estradiol (which were treated as zeros in this comparison), it remains possible that small changes in circulating estradiol levels could have gone undetected, below the sensitivity limit of the assay.
Do Evicted Females Show Evidence of Reproductive Failure?
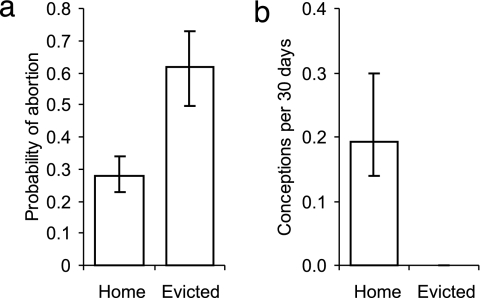
Eviction appeared to interfere substantially with subordinate female reproduction (Fig. 2). Subordinate females that were evicted while pregnant were significantly more likely to abort than those who were not evicted (Fig. 2a; GLMM: χ21 = 6.53, P = 0.011, based on 133 pregnancies carried by 94 subordinates in 15 groups), while controlling for a negative effect of female age (χ21 = 4.56, P = 0.033) and significant seasonal variation (χ23 = 14.12, P = 0.003). Subordinate females were also significantly less likely to conceive while evicted than when within their groups (Fig. 2b; Wilcoxon matched pairs signed-rank test: W = 151, n = 17 females, P < 0.001). Indeed, eviction may effectively block conception (Fig. 2b), because only one evictee conceived in >5,800 female days of eviction monitoring, although evictees frequently copulated with prospecting males from other groups (15).
Fig. 2.
Effect of eviction on subordinate female reproduction. (a) Subordinate females that were evicted during their pregnancy were more likely to abort than those that were not. Bars present predicted means ± 1 SE from the GLMM. (b) Subordinate females were substantially less likely to conceive while evicted than they were when in the group. Bars show medians ± quartiles.
Do Evicted Females Lose Body Weight?
Subordinate females returned from eviction slightly, but significantly, lighter (mean ± SE = 641.3 ± 7.4 g) than on the day of their eviction (mean ± SE = 669.1 ± 10.1 g; paired t test: t = 3.70, n = 38 females that were evicted, P < 0.001), an average loss of 27.8 g or 4.2% body weight. This weight loss may be due to reduced rates of foraging weight gain while evicted (mean ± SE = 2.33 ± 0.60 g/hr) in comparison with the two weeks before eviction (mean ± SE = 4.44 ± 0.63 g/hr; paired t test: t = 2.49, n = 18 females that were evicted, P = 0.023). Paired comparisons of the average weight change per day of subordinate females that were evicted during a given breeding attempt (mean ± SE = −3.53 ± 1.43 g/day) with those of subordinate females that weren’t evicted, over the same time period (mean ± SE = +0.61 ± 0.39 g/day), confirm these weight loss effects (paired t test: n = 13 breeding attempts, t = 3.31, P = 0.006). All weight comparisons excluded pregnant females and those younger than 9 months of age.
Do Dominants Target Reproductive Competitors?
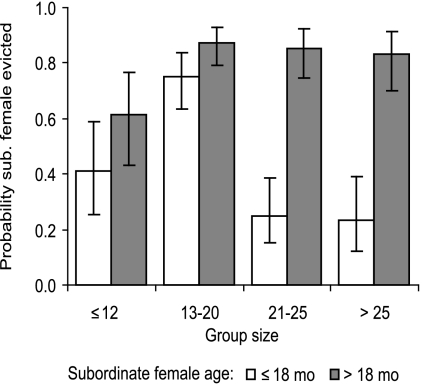
The factors affecting eviction probabilities suggest that dominant females target those subordinates with whom reproductive conflict is most likely. Pregnant dominants only evicted subordinate females of breeding age (those older than 9 months) and, among these, dominants were significantly more likely to evict older females, pregnant females, and those to whom they were more distantly related (Table 1). There was also a significant interaction between subordinate female age and group size (Table 1 and Fig. 3), such that eviction became more likely for females of all ages as group size initially increased. However, at large group sizes (>20 individuals), the eviction probability of younger females declined, suggesting that dominants may be unable to maintain the simultaneous eviction of large numbers of females. Finally, dominant females that were in poorer body condition (lower body weight at conception) were significantly less likely to evict (Table 1).
Table 1.
Factors affecting whether a given subordinate female was evicted during the dominant female’s pregnancy
| Factors | Full model |
Minimal model |
|||
|---|---|---|---|---|---|
| χ2 | df | P | Effect | SE | |
| SF age (<18 mo, >18 mo) | 29.40 | 1 | <0.001 | See Fig. 3 | |
| Average maximum temperature, °C | 19.13 | 1 | <0.001 | −0.19 | 0.045 |
| DF conception weight, g | 10.93 | 1 | 0.001 | 0.016 | 0.0048 |
| SF-DF relatedness (high, low) | 4.81 | 1 | 0.028 | 0.93* | 0.42 |
| SF likely pregnant (yes, no) | 4.37 | 1 | 0.037 | 0.99† | 0.48 |
| Group size (<12, 13–20, 21–25, >25)‡ | 8.20 | 3 | 0.042 | See Fig. 3 | |
| SF age × group size | 7.92 | 3 | 0.048 | See Fig. 3 | |
| DF age, days | 2.92 | 1 | 0.087 | — | — |
| SF average morning weight§ | 1.36 | 1 | 0.24 | — | — |
| Total rainfall, mm | 0.27 | 1 | 0.60 | — | — |
| SF access to unrelated males (yes, no) | 0.00 | 1 | 0.95 | — | — |
| Constant | — | — | — | −1.32 | 0.74 |
Results from a GLMM based on a sample of 527 eviction decisions by 21 dominant females from 12 groups regarding 179 subordinate females over 130 breeding attempts. SF, subordinate female; DF, dominant female.
*Low relatedness > high relatedness.
†Pregnant > nonpregnant.
‡Factorized into four equally represented quartiles.
§Residuals of morning weight on age for all nonpregnant subordinate females.
Fig. 3.
The effect of group size and subordinate female age on the probability that a given subordinate female was evicted during the dominant female’s pregnancy. Bars present predicted means ± 1 SE from the GLMM presented in Table 1.
Discussion
During the latter half of her pregnancy, the dominant female commonly drove subordinate females from the group for an average of three weeks at a time. Evicted subordinates suffered repeated attacks and chases throughout this period and returned to the group only after the dominant had given birth. Evicted subordinates exhibited a 2-fold increase in fecal GC metabolite levels (most likely reflecting substantial activation of the HPA axis), down-regulated reproductive physiology (indicated by reduced pituitary sensitivity to GnRH challenge), and, ultimately, reduced conception rates and increased abortion rates. Dominant females were most likely to evict those subordinates with whom reproductive conflict is expected to be most acute (females of breeding age, older females, pregnant females, and those to whom she was more distantly related). These findings are consistent with the predictions of the stress-related suppression hypothesis and suggest that dominant females use temporary evictions to suppress reproduction by probable competitors when pregnant themselves.
The frequent attacks and chases suffered by evictees provide a likely explanation for their elevated GC levels, although other stressors potentially associated with living away from the main group, such as nutritional stress and increased predation risk (6, 16), are also likely to have played a role. Chronic activation of the HPA axis (and, more specifically, chronic elevation of GC levels) is known to interfere with vertebrate reproductive physiology at a number of levels (5), including down-regulation of the pituitary’s sensitivity to GnRH (which is expected to interfere with ovulation; refs. 5 and 17) and disruption of both oocyte maturation before ovulation and implantation of the conceptus after fertilization (5, 18). Stimulation of the HPA axis throughout the evicted period (an average of 3 weeks) could therefore readily account for the substantially reduced conception rates of evictees, despite their regular copulations with prospecting males from other groups (15). Previous work has demonstrated a role for elevated prolactin, rather than elevated GCs, in the ovulatory block experienced by captive subordinate talapoin monkeys (Miopithecus talapoin) (19), but our findings suggest that mechanisms other than prolactin elevation are likely to be responsible for the apparent infertility of evicted female meerkats, because their prolactin levels remained unchanged. Chronic activation of the HPA axis has also been implicated as a potential disruptor of established pregnancy (18, 20), providing a possible explanation for the elevated abortion rates associated with eviction. Indeed, our analyses probably underestimate the likely impact of evictions on abortion frequency, because pregnancies were detectable only from their fourth week but are most susceptible to disruption in their early stages (18).
It is possible that factors other than activation of the HPA axis, such as loss of body weight, could also have contributed to the down-regulation of subordinate reproduction associated with eviction. Subordinate females did lose a small amount of body weight while evicted from their groups (an average of 27.8 g or 4.2% of body weight), most likely as a consequence of their reduced foraging weight gain rates coupled with costs associated with chronic elevation of GC levels. However, weight loss on this scale is not particularly unusual for meerkats (e.g., top babysitters lose an average of 25 g over the course of the babysitting period; ref. 21), and, although previous analyses suggest that lighter female meerkats conceive less often (13), a loss of only 28 g cannot readily account for the near-total block on conception experienced by evictees.
Pregnant dominants are likely to benefit in two ways from the disruption of subordinate reproduction associated with eviction. First, by suppressing subordinate reproduction, evictions will reduce the chance that the dominant’s own litters [either the one she is carrying while evicting or the one she is likely to conceive postpartum (her modal interbirth interval is 79 days)] will have to compete with those of subordinates for the limited amount of care available in the group. This reduced competition should yield substantial benefits to dominants because the ratio of helpers to pups has a positive influence on pup growth and survival (22). Second, evictions are likely to reduce the threat of infanticide by subordinate females, because subordinate females only kill pups when pregnant themselves (23), and evictions, through their disruptive effects on subordinate reproduction should substantially reduce the likelihood of subordinate pregnancy when the dominant’s litter is born. Evictions may also reduce the opportunity for any subordinates that are still pregnant to commit infanticide (14), because within-group infanticide has not been observed beyond a litter’s fourth day of life (23), and evictees take an average of 3 days to return to the group after the birth of the dominant’s litter.
Dominant female meerkats appear to time and direct their aggression so as to maximize the suppression of probable breeders during critical periods, while minimizing unnecessary persecution (and its likely costs for both parties). Appropriate targeting of aggression may be particularly important in meerkat societies because subordinate females are invariably the dominant’s relatives and helpers (24), so unnecessary costs to subordinates could compromise the dominant’s inclusive fitness. By only evicting when pregnant themselves, dominant females suppressed subordinate reproduction only when it would have otherwise conflicted with their own; subordinate breeding at other times should benefit the dominant through close kinship and group augmentation (22). Dominant females also directed their evictions at those females with whom reproductive conflict was most likely: females of breeding age (those younger than 9 months were never evicted), older females, pregnant females, and those to whom she was more distantly related. Subordinate females who lacked access to breeding partners (unrelated males) in their groups were no less likely to be evicted by dominants, but this apparent lack of discrimination may be adaptive because all females can mate with extragroup males (24). Subordinate females were more likely to be evicted from larger groups, which may reflect the lower cost of losing a helper when group size is large (22). However, younger females were less likely to be evicted from the largest groups than from groups of intermediate size, which may indicate that pregnant dominants cannot sustain the chasing necessary to maintain the eviction of all females at very large group sizes. Indeed, evictions being costly to enforce could also explain why dominants in better condition were more likely to evict. Dominant females in better condition might also have more to gain from evicting, because they produce larger litters of heavier pups and are more likely to conceive a second litter postpartum (13, 25), all of which should increase their payoff from maintaining the suppression of subordinate reproduction.
Together, our findings strongly suggest that stress-related suppression does play a role in the reproductive failure of subordinate female meerkats. Given the lack of evidence for stress-related suppression in other cooperative breeders to date, it is clear that social stress alone cannot explain the reproductive failure of subordinates across such societies (7). However, our findings raise the possibility that, in some cooperative breeders at least, dominants may employ stress-related suppression to guard against lapses in reproductive restraint by their subordinates. Future attempts to investigate whether stress plays a role in the suppression of subordinate reproduction therefore should consider the possibility that dominants may only need to target a subset of their subordinates at critical times.
Materials and Methods
The study was conducted in the South African Kalahari desert (26°59′ S, 21°50′ E) between 1995 and 2003. The study site and climate have been described in refs. 13, 21, and 22. Our study population comprised 15 groups of meerkats that were habituated to close observation. Animals were marked with s.c. transponder chips but were distinguished day to day by unique “hair cuts” on their coats that could be maintained without the need for capture. Study groups were visited at least once every three days to collect group composition, behavioral, and life history data along with fecal samples and body weight data. On emergence from the sleeping burrow at dawn, all individuals were weighed by using an electronic balance. Groups then were followed throughout the morning, with ad lib records kept, using handheld data loggers, of aggressive interactions between group members and with any evicted females encountered. Groups then were typically reweighed after 3 h of foraging so that their rates of foraging weight gain over the morning could be calculated (g/hr). A similar observation period was conducted in the three hours before dusk, when the animals returned to their sleeping burrows. Where possible, similar data were collected by following evictees. The “dominant female” in each group was behaviorally dominant to all other female group members (she displaced them from artificially provided food and marked the substrate with cheek or anal glands at rates ≈10 times higher than those of other females; ref. 14) and was typically older and heavier than all other female group members (13). Pregnancy lasted for 70 days and could be detected from the fourth week after conception due to a swelling of the abdomen and the nipples and a concomitant increase in body weight (13). Birth (or abortion) dates could be identified from a sudden change in the female’s shape and sudden weight loss. Abortion was deemed to have occurred if witnessed or if the female had clearly not carried to full term (e.g., sudden substantial weight loss within 60 days of her last birth or after being visibly pregnant for <4 weeks). Because there were rarely behavioral signs of estrus, conception dates were calculated by backdating 70 days from birth. All research protocols were approved by the University of Pretoria and complied with the Guidelines for the Use of Animals in Research.
Definition and Identification of Eviction.
Eviction is defined as the interaction between the dominant female and a subordinate female that gave rise to the latter spending an extended period away from the group (having to forage and sleep elsewhere but still on the group’s territory). Witnessed evictions comprised a single prolonged attack in which the dominant drove the subordinate from the group and were typically the culmination of escalating aggression during the previous week (14). All evictions were either witnessed (n = 124) or were assumed to have occurred when a subordinate female older than 9 months spent at least 2 days away from the group when the dominant female was known to be pregnant (n = 101). These factors were common to all witnessed evictions, and females did not leave their groups voluntarily for >1 day at a time.
Endocrine Sampling and Analysis.
Subordinate female GC levels were monitored noninvasively by determining the concentrations of GC metabolites in fecal samples. Fecal samples were collected ad libitum and immediately placed on ice and then frozen on return to camp. Steroid metabolites were extracted from fecal samples by using standard methodologies, and GC metabolite concentrations in extracts were determined by using a double-antibody 125I RIA for corticosterone (ICN Biomedicals, Inc., Costa Mesa, CA); see Supporting Text, which is published as supporting information on the PNAS web site, for methodological and validation details.
To monitor subordinate female reproductive physiology, the concentrations of four hormones were determined from blood plasma samples (LH, estradiol, progesterone, and prolactin). Blood samples were collected by using capture and anesthesia protocols described in ref. 26. All samples were collected between 1600 and 2000 hours to minimize diel variation in hormone levels, and no animals were sampled within 12 days of a previous capture. Plasma concentrations of LH were determined by using a previously validated in vitro bioassay based on the production of testosterone by dispersed mouse Leydig cells (11). Assay sensitivity was 2.5 milliunits/ml. Intra- and interassay coefficients of variation were 7% and 12%. Samples were assayed for estradiol by using a previously validated Coat-a-Count Estradiol kit (Diagnostic Products, Los Angeles, CA) (27). Assay sensitivity was 10 pg/ml. Intra- and interassay coefficients of variation were 5.2% and 6.7%. Samples were assayed for progesterone by using a previously validated Coat-a-Count Progesterone kit (Diagnostic Products) (28). Assay sensitivity was 0.1 ng/ml. Intra- and interassay coefficients of variation were 5.7% and 7.9%. Samples were assayed for prolactin by using a previously validated RIA (using rabbit antiserum to human prolactin and canine [125I]iodo-prolactin), (29). Assay sensitivity was 0.05 ng/ml. Intra- and interassay coefficients of variation were 8.3% and 12.6%.
Statistical Analyses.
All analyses were conducted by using GenStat 5 (Release 4.2) (Rothamsted experimental station, Harpenden, U.K.). Several analyses required the use of multivariate statistics for which general(ized) linear models were used. The minimal model was obtained by a reverse stepwise procedure (30). All terms initially were entered into the model and then sequentially dropped until only terms whose elimination would have significantly reduced the explanatory power of the model remained. All two-way interactions were tested, but only those that were significant are presented. The significance of a term was derived by dropping it from the minimal model (if it was part of the minimal model), or by adding it to the minimal model and then dropping it (if it was not part of the minimal model). In each case, a forward stepwise procedure yielded the same minimal model. Several analyses involved repeated measures of the same individual, breeding attempt or group, and in these cases, General(ized) Linear Mixed Models (GLMMs) were used. GLMMs are similar to general(ized) linear models except they allow both fixed and random terms to be defined. Random terms allow the analysis to take account of repeated measures and were included for each level of repeated measurement for which the analysis indicate a nonzero variance component. All statistical tests were two-tailed.
Do Evicted Females Show Elevated GC Levels?
To assess the effect of eviction on a female’s circulating GC levels, fecal samples were collected from subordinate adult females, both in their groups and while evicted, during the peak conceptive season (June to January). Pregnant females were excluded from the analysis because GC excretion typically varies with pregnancy stage (17). The effect of eviction on fecal GC metabolite concentrations was assessed by using a GLMM after a natural logarithm transformation normalized the data. The analysis used a data set of 188 fecal GC measures (98 within group and 90 evicted) from 58 subordinate females from 10 groups, and repeated measures of individuals and groups were controlled by fitting both as random factors in the model. In addition to whether the female was evicted, the analysis controlled for her age, rainfall, temperature, and sample collection time (a.m. or p.m.).
Do Evicted Females Show Down-Regulated Reproductive Physiology?
One way in which chronic stress can compromise fertility is by down-regulating the pituitary’s sensitivity to GnRH, a hormone released by the hypothalamus to stimulate the release of LH by the pituitary (5, 17). We assessed pituitary sensitivity to GnRH by conducting a standardized GnRH challenge and comparing the pre- and postchallenge concentrations of LH in blood plasma (as per ref. 11). In each case, the prechallenge blood sample was collected 5–10 min after capture, and the GnRH challenge was administered immediately afterward (a single s.c. injection of 2 μg of exogenous GnRH in 200 μl of physiological saline). The postchallenge blood sample was drawn 20 min after GnRH administration. To investigate the effect of eviction on pituitary sensitivity, GnRH challenges were conducted (i) before eviction (7–45 days after the dominant conceived) and (ii) while evicted (7–58 days after eviction, but before the dominant female gave birth). Blood samples from these periods were also used to assess the effect of eviction on plasma levels of estradiol, progesterone, and prolactin. The number of subordinate females sampled in the pre- and posteviction contexts, respectively, were n = 18 and 9 for GnRH challenges, n = 13 and 12 for estradiol, n = 9 and 9 for progesterone, and n = 16 and 11 for prolactin. Sample sizes vary because it was not possible to assay all samples for all hormones, and because prolactin was measured only in those samples collected within 4–6 min of capture to minimize the effects of capture stress on prolactin levels (29). All blood samples (including GnRH challenges) were collected from nonpregnant, subordinate adult females during the peak conceptive season (June to January).
Do Evicted Females Show Evidence of Reproductive Failure?
To assess the effect of eviction on abortion rates, all subordinate pregnancies were classified as either “evicted” or “home” depending on whether the female had been evicted during her pregnancy. The effect of this factor was then tested in a GLMM with a binomial response describing whether the litter was born (0) or aborted (1) by using a data set of 133 pregnancies carried by 94 subordinate females from 15 groups. Repeated measures of groups and individuals were controlled by fitting both as random factors in the model. To ensure that any difference in abortion rate between contexts could not have arisen simply from differential information on pregnancy fate in the two contexts, we included only those pregnancies for which the female (evicted or not) was observed both on the day before and the day after the end of the pregnancy. In addition to whether the female was evicted during the pregnancy, her age and weight at conception, as well as group size, temperature, rainfall, and season (January to March, April to June, July to September, and October to December) also were fitted to the model as fixed terms.
To investigate the effects of eviction on female conception rates, the conception rates of each subordinate adult female were calculated for two contexts: within their groups and whilst evicted. First, all subordinate conception dates known to within two weeks were classified as having occurred whilst the female was either in her group or evicted from it. Where conception dates were not known precisely, the female was considered to have conceived while evicted if she was evicted for one or more of the possible conception days, thereby keeping the analysis conservative by overestimating evictee conception rates. A conception rate then was calculated for each female in each context by dividing the number of times that she conceived in each context by the total number of possible conception days (nonpregnant days) spent in that context while being monitored as an adult. To ensure that all females in the comparison had experienced a substantial period of exile in which to potentially conceive, females were included only if they had spent a total of >30 days evicted during their adult life (this restriction of the data set did not qualitatively affect the results). To avoid underestimating the within-group conception rates, the nonconceptive season (February to May) was excluded from the analysis, because females were rarely evicted during this period. This approach yielded a paired sample of 17 subordinate females from eight groups.
Do Evicted Females Lose Body Weight?
To assess the effect of eviction on subordinate female rates of weight gain while foraging (g/hr), their foraging weight gain rates while evicted were compared with those during the two weeks before the same eviction (n = 18 females). Average values were taken when multiple measures of foraging weight gain were available within a context for a given eviction and again when data for multiple evictions were available for a given subordinate female. To assess the effect of eviction on female body weight, their body weights on the morning of the day of eviction were compared with those on the morning of the day of return from the same eviction (n = 38 females). Again, where measures were available for multiple evictions of a given female, they were averaged to yield a single pair of data points per female. Finally, the weight changes of evictees were compared with those of subordinate females that were not evicted during the same breeding attempt. For each breeding attempt, the average change in body weight per day was calculated for each evictee (by dividing her change in weight from eviction day to return day by the duration of her eviction) and for each subordinate female >9 months of age that was not evicted [by dividing her change in weight during the final 20 days of the dominant female’s pregnancy (the median eviction period) by 20]. Where values for multiple evictees or nonevictees were available for a given breeding attempt, values were averaged, yielding a data set of 11 breeding attempts for which paired weight change data were available for both evictees and nonevictees. To avoid problems arising from weight changes associated with pregnancy, data from pregnant females were excluded from all analyses.
Do Dominants Target Reproductive Competitors?
A GLMM with binomial error structure was used to investigate the factors influencing whether a subordinate female was evicted by her pregnant dominant. For each dominant female pregnancy, all subordinate females in the group >9 months of age (because females younger than this were never evicted) were classified as either evicted (1) or not evicted (0), and this binomial term was set as the model response. The analysis used a sample of 527 eviction decisions (evict or not), by 21 dominant females from 12 groups, regarding 179 subordinate females over 130 breeding attempts. Group and breeding attempt were fitted as random terms to control for repeated measures. The following fixed effects were tested in the model: subordinate female characteristics [age in days when the dominant female conceived, average morning weight when in the group during the dominant’s pregnancy, relatedness to the dominant female (r = 0.5 for sisters or daughters, or r < 0.5), access to unrelated males in the group (yes or no), and pregnancy status 3 weeks before dominant female parturition; the median timing of eviction]; dominant female characteristics (age and weight at conception); group size (excluding pups); and environmental characteristics (temperature and rainfall).
Supplementary Material
Acknowledgments
We thank those at the Mammal Research Institute and our volunteers for field assistance, Northern Cape Conservation for research permission, N. Wielebnowski and S. Bircher at Brookfield and St. Louis Zoological Parks for assistance with physiological validations, E. B. Keverne for suggestions during the study, and three anonymous referees for their valuable contributions to the manuscript. This research was funded by the Natural Environment Research Council; the Biotechnology and Biological Sciences Research Council; the Royal Society, UK; and Magdalene College (Cambridge, U.K.).
Abbreviations
- GC
glucocorticoid
- GnRH
gonadotropin-releasing hormone
- IQR
interquartile range
- GLMM
general(ized) linear mixed model.
Footnotes
Conflict of interest statement: No conflicts declared.
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Koenig W. D., Dickinson J. L. Ecology and Evolution of Cooperative Breeding in Birds. Cambridge, U.K.: Cambridge Univ. Press; 2004. [Google Scholar]
- 2.Wingfield J. C., Hegner R. E., Lewis D. M. J. Zool. 1991;225:43–58. [Google Scholar]
- 3.Keverne E. B., Meller R. E., Eberhart A. In: Advanced Views in Primate Biology. Chiarelli A. B., Corruccini R. S., editors. Berlin: Springer; 1982. pp. 81–94. [Google Scholar]
- 4.Schoech S. J., Mumme R. L., Moore M. C. Condor. 1991;93:354–364. [Google Scholar]
- 5.Pottinger T. G. In: Stress Physiology in Animals. Balm P. H. M., editor. Sheffield, U.K.: Sheffield Academic; 1999. pp. 130–177. [Google Scholar]
- 6.von Holst D. Adv. Study Behav. 1998;27:1–131. [Google Scholar]
- 7.Creel S. Trends Ecol. Evol. 2001;16:491–497. [Google Scholar]
- 8.Abbott D. H., Saltzman W., SchultzDarken N. J., Smith T. E. Ann. N.Y. Acad. Sci. 1997;807:219–238. doi: 10.1111/j.1749-6632.1997.tb51923.x. [DOI] [PubMed] [Google Scholar]
- 9.Faulkes C. G., Abbott D. H. In: Cooperative Breeding in Mammals. Solomon N. G., French J. A., editors. Cambridge, U.K.: Cambridge Univ. Press; 1997. pp. 302–334. [Google Scholar]
- 10.Wasser S. K., Barash D. P. Q. Rev. Biol. 1983;58:513–538. doi: 10.1086/413545. [DOI] [PubMed] [Google Scholar]
- 11.O’Riain M. J., Bennett N. C., Brotherton P. N. M., McIlrath G., Clutton-Brock T. H. Behav. Ecol. Sociobiol. 2000;48:471–477. [Google Scholar]
- 12.Heinsohn R. G. Am. Nat. 1991;137:864–881. [Google Scholar]
- 13.Clutton-Brock T. H., Brotherton P. N. M., Russell A. F., O’Riain M. J., Gaynor D., Kansky R., Griffin A., Manser M., Sharpe L., McIlrath G. M., et al. Science. 2001;291:478–481. doi: 10.1126/science.291.5503.478. [DOI] [PubMed] [Google Scholar]
- 14.Clutton-Brock T. H., Brotherton P. N. M., Smith R., McIlrath G. M., Kansky R., Gaynor D., O’Riain M. J., Skinner J. D. Proc. R. Soc. London Ser. B; 1998. pp. 2291–2295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Young A. J. Ph.D. thesis. Cambridge, U.K.: University of Cambridge; 2003. [Google Scholar]
- 16.Woodley C. M., Peterson M. S. Oecologia. 2003;136:155–160. doi: 10.1007/s00442-003-1236-1. [DOI] [PubMed] [Google Scholar]
- 17.Johnson M. H., Everitt B. J. Essential Reproduction. Oxford: Blackwell Science; 1995. [Google Scholar]
- 18.de Catanzaro D., Macniven E. Neurosci. Biobehav. Rev. 1992;16:43–53. doi: 10.1016/s0149-7634(05)80050-8. [DOI] [PubMed] [Google Scholar]
- 19.Bowman L. A., Dilley S. R., Keverne E. B. Nature. 1978;275:56–58. doi: 10.1038/275056a0. [DOI] [PubMed] [Google Scholar]
- 20.Arck P. Am. J. Reprod. Immunol. 2001;46:117–123. doi: 10.1111/j.8755-8920.2001.460201.x. [DOI] [PubMed] [Google Scholar]
- 21.Clutton-Brock T. H., Gaynor D., Kansky R., MacColl A. D. C., McIlrath G., Chadwick P., Brotherton P. N. M., O’Riain J. M., Manser M., Skinner J. D. Proc. R. Soc. Lond. Ser. B; 1998. pp. 185–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Clutton-Brock T. H., Russell A. F., Sharpe L. L., Brotherton P. N. M., McIlrath G. M., White S., Cameron E. Z. Science. 2001;293:2446–2449. doi: 10.1126/science.1061274. [DOI] [PubMed] [Google Scholar]
- 23.Young A. J., Clutton-Brock T. H. Biol. Lett. 2006 doi: 10.1098/rsbl.2006.0463. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Griffin A. S., Pemberton J. M., Brotherton P. N. M., McIlrath G., Gaynor D., Kansky R., O’Riain J., Clutton-Brock T. H. Behav. Ecol. 2003;14:472–480. [Google Scholar]
- 25.Russell A. F., Brotherton P. N. M., McIlrath G. M., Sharpe L. L., Clutton-Brock T. H. Behav. Ecol. 2003;14:486–492. [Google Scholar]
- 26.Young A. J., Carlson A. A., Clutton-Brock T. H. Anim. Behav. 2005;70:829–837. [Google Scholar]
- 27.Carlson A. A., Young A. J., Russell A. F., Bennett N. C., McNeilly A. S., Clutton-Brock T. Horm. Behav. 2004;46:141–150. doi: 10.1016/j.yhbeh.2004.01.009. [DOI] [PubMed] [Google Scholar]
- 28.Russell A. F., Carlson A. A., McIlrath G. M., Jordan N. R., Clutton-Brock T. Evolution (Lawrence, Kans.) 2004;58:1600–1607. doi: 10.1111/j.0014-3820.2004.tb01739.x. [DOI] [PubMed] [Google Scholar]
- 29.Carlson A. A., Nicol L., Young A. J., Parlow A. F., McNeilly A. S. Gen. Comp. Endocrinol. 2003;130:148–156. doi: 10.1016/s0016-6480(02)00610-x. [DOI] [PubMed] [Google Scholar]
- 30.Crawley M. J. Statistical Computing. Chichester, U.K.: Wiley; 2002. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.