Abstract
Animal studies have shown that the brain is an insulin-responsive organ and that central nervous insulin resistance induces obesity and disturbances in glucose metabolism. In humans, insulin effects in the brain are poorly characterized. We used a magnetoencephalography approach during a two-step hyperinsulinemic euglycemic clamp to (i) assess cerebrocortical insulin effects in humans, (ii) compare these effects between 10 lean and 15 obese subjects, and (iii) test whether the insulin receptor substrate (IRS)-1 Gly972Arg polymorphism in the insulin-signaling cascade modifies these effects. Both spontaneous and stimulated (mismatch negativity) cortical activity were assessed. In lean humans, stimulated cortical activity (P = 0.046) and the beta and theta band of spontaneous cortical activity (P = 0.01 and 0.04) increased with insulin infusion relative to saline. In obese humans, these effects were suppressed. Moreover, the insulin effect on spontaneous cortical activity correlated negatively with body mass index and percent body fat (all r < −0.4; all P < 0.05) and positively with insulin sensitivity of glucose disposal (theta band, r = 0.48, P = 0.017). Furthermore, insulin increased spontaneous cortical activity (beta band) in carriers of wild-type IRS-1, whereas, in carriers of the 972Arg allele, this insulin effect was absent (P = 0.01). We conclude that, in lean humans, insulin modulates cerebrocortical activity, and that these effects are diminished in obese individuals. Moreover, cerebrocortical insulin resistance is found in individuals with the Gly972Arg polymorphism in IRS-1, which is considered a type 2 diabetes risk gene.
Keywords: glucose metabolism, insulin resistance, magnetoencephalography, mismatch negativity, type 2 diabetes
The human brain has been traditionally regarded as an insulin-insensitive organ. However, there is now growing evidence that insulin signaling might be an important modulator of several functions of the brain. The insulin receptor and other components of the insulin-signaling chain, such as the insulin receptor substrate (IRS)-1 are ubiquitously expressed throughout the brain in animals and humans with particularly high concentrations in the hypothalamus, the hippocampus, and the cerebral cortex (1–3). Early work in animals suggested that insulin acts in the CNS and controls food intake and body weight (4). Furthermore, it was clearly shown that insulin crosses the blood–brain barrier (5) and, when given directly to the brain, suppresses food intake (6, 7) and mediates peripheral metabolic effects (8). Most important for the understanding of central nervous insulin function was the observation that brain-specific deletion of the insulin receptor in mice resulted in hyperphagia, obesity, and metabolic insulin resistance (9).
In mice, insulin effects have been intensively studied in the hypothalamus. However, it is noteworthy that insulin receptors are also abundant in the cerebral cortex (2). Furthermore, intracerebroventricular insulin administration was associated with improved cognitive function in rodents (10), suggesting that insulin acts in the cerebral cortex. Glucose utilization of the CNS has been intensively studied with positron emission tomography in humans and no effect of hyperinsulinemia has been found (11–13). In contrast, it has recently been shown that intranasal administration and i.v. bolus injection of insulin influences neuronal activity of the cerebral cortex (measured by electroencephalography), memory, and body weight (14–17). Moreover, in patients with Alzheimer's disease, administration of insulin has been shown to improve learning and memory (18). However, it is still unclear whether cerebrocortical insulin resistance exists in humans and, if so, whether it is related to peripheral insulin resistance and obesity.
The assessment of insulin effects in the human CNS is difficult, and all available methods have major limitations. Some techniques, such as functional magnetic resonance imaging, have limited temporal resolution, and others focus predominantly on metabolic processes (such as measurement of glucose uptake by positron emission tomography) as opposed to neuronal activity. Magnetoencephalography (MEG) measures magnetic fields generated by the electrical activity of cortical neurons. MEG provides high temporal and spatial information of brain activity, especially for cerebral cortex activation, and is, therefore, well suited for noninvasive measurement of neuronal activity in the human cerebral cortex. Moreover, by using MEG paradigms, such as mismatch negativity (MMN), cortical information processing can be quantified.
To determine whether the cerebral cortex is directly influenced by systemic insulin, we performed MEG during a two-step hyperinsulinemic euglycemic clamp. First, we aimed to develop quantitative parameters for assessing cerebrocortical insulin effects in humans. Second, we compared these effects between lean and overweight individuals. Third, we tested whether genetically induced alteration in the insulin-signaling cascade influences these effects. As a model, we used the prevalent IRS-1 Gly972Arg polymorphism, which was found to impair insulin signaling along the p85/PIP3-kinase pathway (19–21).
Results
Insulin Effect in Lean Subjects.
During the hyperinsulinemic euglycemic clamp, plasma insulin concentrations increased from 38 ± 3 pM in the basal state by 2-fold in the first step (83 ± 6 pM; P < 0.001) and by 9-fold in the second step (351 ± 23 pM; P < 0.001), whereas, during the saline infusion as a control, insulin slightly decreased from 39 ± 3 to 26 ± 3 pM. Plasma glucose was not different at baseline between the saline and the insulin experiment (4.7 ± 0.1 and 4.7 ± 0.1 mmol/liter; P = 0.80). Due to the experimental design (in which we targeted a glucose concentration of 5 mmol/liter during the clamp to avoid hypoglycemia), plasma glucose was slightly higher during the second step of the hyperinsulinemic euglycemic clamp (4.9 ± 0.1 mmol/liter) than during the saline experiment (4.5 ± 0.1 mmol/liter; P = 0.03).
Fig. 1A shows the topographic map of mismatch fields by MEG in the basal state of the insulin (Left) and saline (Right) experiment. The global measure of basal MMN (root mean square over all MEG channels) was not different between the saline and the insulin experiment (P = 0.15). Accordingly, the topographic difference map shows no activation sites of MMN (Fig. 1 B Left and C). During the first step of the clamp (insulin concentrations 2-fold over basal), MMN did not change significantly (P = 0.55 vs. saline). However, as the insulin concentration was increased by 9-fold in the second step of the clamp, the difference map showed an activation of mismatch fields bilaterally in the auditory cortex accompanied by a higher global MMN in the insulin vs. saline experiment (7.37 ± 0.07 fT vs. 7.20 ± 0.09 fT; P = 0.046). This difference implies enhanced information processing during insulin stimulation.
Fig. 1.
Topographic map for the grand averages of the MMN fields. Data from 151 sensors are shown. The orientation of the head is given by the following letters: f, frontal; l, left; r, right. The viewer looks from above the head. (A) Basal recordings of MMN fields (see Methods) before starting the insulin (Left) or saline (Right) experiment. Data are from eight lean subjects (Table 4). (B) Topographic difference map for the grand averages of the MMN fields for the insulin experiment minus the saline experiment (lean subjects). The difference of MMN activity between the insulin and the saline experiment is interpolated and plotted two-dimensionally in a color-coded manner. Lack of color implies a difference of zero. (Left) Basal recording. (Right) Recorded during second step of insulin infusion (1.0 milliunit/kg per min). (C) Topographic difference map for the grand averages of the MMN fields for the insulin experiment minus the saline experiment (obese subjects). (Left) Basal recording. (Right) Recorded during second step of insulin infusion (1.0 milliunit/kg per min).
In addition to the mismatch fields, we found significant effects in the beta and theta bands of spontaneous MEG data for the different experiments, which remained after correction for multiple comparisons in the different frequency bands. In the basal state, all measures of cortical activity were not significantly different between the insulin and saline experiment (all P > 0.2). However, beta activity increased by 10 ± 9 fT during the insulin experiment and decreased during the saline condition by 17 ± 7 fT (repeated-measures ANOVA, P = 0.02, interaction insulin vs. saline x time). Accordingly, theta activity increased by 54 ± 14 fT during insulin infusion and decreased by 49 ± 19 fT during saline infusion (repeated-measures ANOVA, P < 0.001) (Fig. 2). These observations may imply increased cortical information processing and improved memory function due to insulin stimulation.
Fig. 2.
MEG parameters and plasma insulin levels before (basal) and during infusion of insulin or saline (first and second step) in 10 lean subjects. Shown are beta activity relative to baseline (A), theta activity relative to baseline (B), glucose infusion rate (C), and plasma insulin (D). ∗, P < 0.05, paired t test.
Relation of Cerebrocortical Insulin Effects to Obesity and Peripheral Glucose Metabolism.
To determine whether the insulin effect on cerebrocortical activity would be altered in conditions associated with decreased insulin signaling, two models were explored: obesity and IRS-1 Gly972Arg polymorphism.
Despite significantly higher steady-state insulin concentrations in the obese group during the hyperinsulinemic euglycemic clamp, the glucose infusion rate was significantly lower, indicating insulin resistance (Fig. 3 C and D). This difference is also evident from the significantly lower insulin sensitivity index derived from the hyperinsulinemic euglycemic clamp (Table 1). In both groups, insulin increases ≈9-fold over basal with the high-dose insulin infusion step (lean, 10.2 ± 1.3-fold; obese, 9.2 ± 0.9-fold; P = 0.52). C-peptide decreased slightly but not significantly (basal, 261 ± 32; second step, 249 ± 28 pM; P = 0.57), with no difference between the two groups (P = 0.22).
Fig. 3.
Comparison of the insulin effect (expressed as the difference from the saline experiment) between lean and obese subjects. Shown are beta activity (A), theta activity (B), glucose infusion rate (C), and plasma insulin (D). ∗, P < 0.05, paired t test.
Table 1.
Peripheral insulin effects in lean and obese subjects (hyperinsulinemic euglycemic clamp)
| Time period | Steady-state plasma glucose, mmol/liter |
Steady-state plasma insulin, pmol/liter |
Glucose infusion rate, μmol·kg−1·min−1 |
Insulin sensitivity, μmol·kg−1·min−1·pmol−1·liter |
||||
|---|---|---|---|---|---|---|---|---|
| Lean | Obese | Lean | Obese | Lean | Obese | Lean | Obese | |
| Basal | 4.68 ± 0.08 | 4.75 ± 0.10 | 38 ± 3 | 70 ± 10* | – | – | – | – |
| First step | 5.13 ± 0.09 | 5.00 ± 0.09 | 79 ± 6 | 149 ± 16† | 9.6 ± 1.2 | 5.5 ± 0.6† | 0.12 ± 0.01 | 0.04 ± 0.01‡ |
| Second step | 4.87 ± 0.08 | 4.60 ± 0.10 | 351 ± 23 | 547 ± 43† | 44.8 ± 4.0 | 23.4 ± 2.7‡ | 0.13 ± 0.01 | 0.06 ± 0.01‡ |
∗, P < 0.05;
†, P < 0.01;
‡, P < 0.001.
In contrast to the increase of spontaneous cortical activity in the lean group during insulin infusion, in obese subjects there was no effect of insulin on beta band activity (P = 0.9) and even a decrease of theta band activity (P = 0.04). The statistical parametric mapping revealed a significant group X level (i.e., basal, first step, second step) interaction (P < 0.001) in the theta power band (Fig. 3). Similarly, the insulin-induced increase in MMN in the lean subjects (Fig. 1B) was absent in the obese subjects (Fig. 1C, P = 0.3). As expected from the differences between lean and obese subjects, the insulin-induced change in theta activity was closely correlated with body mass index (BMI) and percent body fat (Fig. 4 A + B). Moreover, we found a positive correlation of theta activity with insulin sensitivity of glucose disposal (Fig. 4C).
Fig. 4.
Correlation between insulin-induced change in theta activity (i.e., cortical insulin sensitivity) and BMI (r = −0.74, P < 0.001) (A), percent body fat (r = −0.65, P = 0.001) (B), and metabolic insulin sensitivity (insulin-stimulated glucose disposal, r = 0.48, P = 0.017) (C).
Similarly, the insulin-induced change in beta activity was negatively correlated with BMI (r = −0.48, P = 0.02) and percent body fat (adjusted for gender difference, r = −0.43, P = 0.04). However, no correlation was found between the change in beta activity and insulin sensitivity of glucose disposal (P = 0.27).
Effects of the Gly972Arg Polymorphism in IRS-1.
Multiple previous studies in vitro (19–21) and in vivo (22–25) have shown that the Gly972Arg polymorphism is linked to decreased insulin signaling and insulin secretion and an increased risk of type 2 diabetes. For analysis of the genotype effect in IRS-1, we compared 11 carriers of the arginine allele [10 heterozygous (Gly/Arg) and 1 homozygous (Arg/Arg)] with 11 wild-type (Gly/Gly) subjects. The genotype groups were well matched for anthropometric and metabolic parameters (see Table 2). During the clamp procedures, they show similar steady-state plasma glucose and plasma insulin levels, as well as similar glucose infusion rates and insulin sensitivity (Table 3).
Table 2.
Subjects' characteristics in IRS-1 Gly972Arg group
| Parameter | Gly/Gly | X/Arg | P |
|---|---|---|---|
| No. | 11 | 11 | – |
| Sex, male/female | 5/6 | 5/6 | 1.0 |
| Age, years | 31 ± 3 | 33 ± 3 | 0.85 |
| Weight, kg | 72.8 ± 4.6 | 71.3 ± 5.0 | 0.82 |
| BMI, kg/m2 | 24.42 ± 1.37 | 24.43 ± 1.38 | 0.99 |
| Body fat, % | 22.7 ± 2.5 | 23.6 ± 2.5 | 0.81 |
| Waist–hip ratio | 0.859 ± 0.028 | 0.855 ± 0.030 | 0.93 |
| Fasting glucose, mmol/liter | 4.96 ± 0.09 | 4.83 ± 0.13 | 0.44 |
| 2-hr glucose, mmol/liter* | 5.95 ± 0.43 | 5.73 ± 0.37 | 0.70 |
| Fasting insulin, pmol/liter | 46 ± 7 | 53 ± 7 | 0.47 |
| 2-hr insulin, pmol/liter* | 367 ± 84 | 398 ± 100 | 0.81 |
*Oral glucose tolerance test. For all parameters, the mean and standard error of the mean are shown.
Table 3.
Peripheral insulin effects in IRS-1 Gly/Gly and X/Arg subjects (hyperinsulinemic euglycemic clamp)
| Time period | Steady-state plasma glucose, mmol/liter |
Steady-state plasma insulin, pmol/liter |
Glucose infusion rate, μmol·kg−1·min−1 |
Insulin sensitivity, μmol·kg−1·min−1pmol−1·liter |
||||
|---|---|---|---|---|---|---|---|---|
| Gly/Gly | X/Arg | Gly/Gly | X/Arg | Gly/Gly | X/Arg | Gly/Gly | X/Arg | |
| Basal | 4.73 ± 0.06 | 4.50 ± 0.17 | 47 ± 5 | 41 ± 10 | – | – | – | – |
| First step | 5.09 ± 0.09 | 5.12 ± 0.13 | 97 ± 12 | 119 ± 18 | 7.8 ± 1.1 | 7.5 ± 1.1* | 0.10 ± 0.02 | 0.08 ± 0.02† |
| Second step | 4.72 ± 0.08 | 4.88 ± 0.19 | 424 ± 32 | 406 ± 40 | 39.3 ± 5.0 | 29.8 ± 3.9‡ | 0.10 ± 0.02 | 0.09 ± 0.02§ |
∗, P = 0.86;
†, P = 0.44;
‡, P = 0.24;
§, P = 0.56.
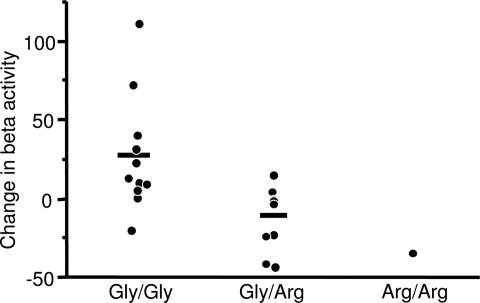
For comparisons between the genotype groups, the MEG parameters were again treated as the difference of the insulin and saline condition. As shown in Fig. 5, the stimulation of beta band activity by insulin infusion compared with saline was markedly reduced in the carriers of the arginine allele (ANOVA, P = 0.01). There was no different stimulation of theta band or MMN activity between Gly/Gly and X/Arg individuals (repeated-measures ANOVA, P > 0.2).
Fig. 5.
Insulin-induced change in beta activity (minus saline-derived change) in the Gly/Gly, Gly/Arg, and Arg/Arg genotypes (ANOVA, P = 0.01).
Discussion
Animal studies have provided compelling evidence that insulin acts in the brain and in peripheral tissues to regulate body weight and control glucose production by the liver (8, 9). Similar effects have been suggested by human studies (15). In the present study, we detected insulin-induced changes in cerebrocortical activity in lean humans by using MEG, whereas, in obese humans, these effects were not detectable. Cerebrocortical insulin action was positively correlated with peripheral insulin sensitivity and negatively correlated with measures of obesity. In addition, the insulin effects on spontaneous cortical activity were reduced in a model of genetically reduced insulin signaling by way of the IRS-1 pathway.
We studied both spontaneous neuronal activity (beta and theta band activity) as well as evoked neuronal activity (MMN, auditory-evoked mismatch fields) during a two-step hyperinsulinemic euglycemic clamp. To control for daytime variations in cerebrocortical activity (26) and fatigue or repetition effects, we performed a saline experiment in random order and interpreted the change during insulin infusion in relation to saline-derived changes. Analysis of the different frequency bands of spontaneous MEG was performed by correcting for multiple comparisons to exclude spurious results.
MMN is a robust parameter that is independent of alertness and attentiveness and is generated directly by changes in neuronal activity (27). Therefore, effects on this parameter are of particular value (27, 28) and represent a specific modulation of cerebrocortical function by insulin. Moreover, the two-dimensional mapping clearly shows that the main effect is located in the primary auditory cortex. The increase in auditory MMN could be a sign of higher capability in discrimination of a deviant tone (29). The auditory evoked cerebrocortical response has been shown to be altered in type 2 diabetes (30, 31) and after intranasally administered insulin (17). Thus, the effect of insulin on MMN clearly shows that insulin modulates brain function in humans.
In addition, it is well known that enhanced theta activity is related to increased memory performance (32) in agreement with the idea that theta activity is a signature of memory consolidation. Moreover, increased activity in higher frequency bands like the beta band is commonly associated with increased cortical processing activity (33). In elderly individuals, type 2 diabetes has been shown to contribute to the decline in cognitive function (34), and, in Alzheimer's disease, insulin therapy improves memory performance (18). Furthermore, there is convincing evidence for decreased central nervous insulin signaling in the pathogenesis of Alzheimer's disease (35). The direction of change of beta and theta activity under insulin is generally associated with better cognitive performance and is in accordance with the finding that the central nervous insulin signal is disturbed in Alzheimer's disease.
Insulin-induced changes in cerebrocortical activity in lean subjects are likely to be transmitted directly through the neuronal insulin signaling cascade. In mice, we could show that human insulin activates cerebrocortical and hypothalamic insulin signaling molecules, such as insulin receptor, IRS, or PI3-kinase, after bolus injection to the cava vein within 2 min (36). Indirect mechanisms, such as insulin-induced increased blood flow or increased brain glucose metabolism, are unlikely to play a role. In contrast to insulin-induced hypoglycemia, there is no evidence for increase of cerebral blood flow by insulin under euglycemic conditions (11, 37). Moreover, an increase of cerebral blood flow by vasoactive substances does not change cerebrocortical activity measured by electroencephalography recorded from the cortical surface (38). The insulin effect on brain glucose uptake has been studied with positron emission tomography in humans. A permissive role of basal insulin concentrations for global brain glucose uptake has been demonstrated (39). However, hyperinsulinemia does not stimulate glucose uptake of the brain compared with fasting insulin levels (11–13).
Using the change during the insulin experiment minus the change during the saline experiment, we compared a group of obese individuals with the lean control group. In the obese group, the insulin effect was much smaller or absent. The blunted cerebrocortical insulin effects were seen despite substantially higher steady-state insulin concentrations in the obese group. The higher insulin concentrations may be the result of decreased insulin clearance in this group. Furthermore, a relative overestimation of the insulin dose in the obese group, due to calculating the insulin dose based on body weight instead of body surface area, contributes to this difference as well. The direction of the difference of insulin levels between the two groups makes our findings even more compelling and strongly suggests the presence of cerebrocortical insulin resistance in obese subjects.
The mechanism of cerebrocortical insulin resistance in obese humans could reside at various levels. It might be located at the transport step of insulin across the blood–brain barrier, which appears to be a receptor-mediated saturable process (40). It could also be an intraneuronal defect in the insulin signaling cascade of either a primary (i.e., genetic) or secondary (i.e., due to an obesity-related factor) nature.
To test the hypothesis that primary, genetically determined cerebrocortical insulin resistance exists in humans, we used a common polymorphism in the IRS-1 gene as a model. Genetic variants in IRS-1, which is widely distributed in the brain (1), represented prime candidates for impairing the insulin-signaling cascade. In vitro studies have shown that this variant impairs the ability of insulin to activate the IRS-1/PI3-kinase/Akt/GSK-3 signaling pathway, thus leading to defects in glucose transport, glucose transporters translocation, and glycogen synthesis (19–21). In humans, the common Gly972Arg polymorphism in IRS-1 is associated with type 2 diabetes (21). Although a clear impairment of insulin secretion has been shown, the results on insulin sensitivity are controversial in humans (22–25, 41). This phenomenon has been attributed to redundant signaling pathways. Because the reduced signaling by way of IRS-1 is widely compensated by the IRS-2 pathway in skeletal muscle, one may speculate that this compensation mechanism is tissue-specific and does not exist in neuronal cells.
The genotype groups in IRS-1 were carefully matched for body weight, age, and gender to exclude obesity-related differences. Our data suggest that, in the human cerebral cortex, this polymorphism is associated with reduced neuronal response to insulin. This finding can be interpreted as a proof of principle for the existence of a primary, genetically determined cerebrocortical insulin resistance. It is of note that we found significant insulin effects for different frequency bands for various experimental groups. Comparing lean and obese subjects, we show insulin-induced increments in beta and theta activity in the lean subjects, which were absent in the obese, whereas for the IRS-1 Gly972Arg polymorphism, we only could determine differences in beta band activity. In any case, the difference between the IRS-1-dependent and the obesity-dependent pattern of central nervous insulin resistance suggests that different mechanisms lead to cerebral insulin resistance.
In summary, in lean, healthy humans, insulin modulates cerebrocortical activity. This modulation occurs both in spontaneous and auditory-stimulated (MMN) activity. In overweight but otherwise healthy individuals, the cerebrocortical insulin effects are absent. Cerebrocortical insulin action correlates with BMI and whole-body insulin action. Obesity-related factors might therefore play a common role in the induction not only of peripheral insulin resistance but also of central nervous insulin resistance. Finally, we show that genetically determined alterations of the insulin-signaling pathway also result in reduced neuronal response to insulin, however, with a somewhat different pattern.
Taken together, these findings suggest that genetically determined and obesity-related cerebrocortical insulin resistance exists in humans, opening the possibility that, just as shown in animal models, central nervous insulin resistance might be not only a consequence but also a starting point for the development of obesity and possibly of type 2 diabetes. If this hypothesis can be further substantiated, improvement of CNS insulin action may become a therapeutic paradigm to prevent obesity and type 2 diabetes.
Methods
Human Subjects.
We studied 10 lean and 15 overweight or obese subjects who were otherwise healthy and whose glucose tolerance was considered normal according to World Health Organization criteria. To ensure that overweight was attributable to elevated fat mass, a cutoff of 21% and 27% body fat was used in men and women, respectively. This cutoff corresponds to a BMI of ≈25 kg/m2. The overweight and obese subjects collectively will be referred to as “obese.” A BMI >35 kg/m2 and/or psychiatric disorders represented exclusion criteria. The subject characteristics are shown in Table 4.
Table 4.
Subjects' characteristics in lean vs. obese group
| Parameter | Lean group | Obese group | P |
|---|---|---|---|
| No. | 10 | 15 | – |
| Sex, male/female | 4/6 | 7/8 | 0.8 |
| Age, years | 26 ± 1 | 33 ± 2 | 0.06 |
| Weight, kg | 65.2 ± 4.4 | 88.3 ± 3.3 | <0.001 |
| BMI, kg/m2 | 21.1 ± 0.8 | 29.7 ± 0.7 | <0.001 |
| Body fat, % | 17 ± 2 | 32 ± 2 | <0.001 |
| Waist–hip ratio | 0.82 ± 0.02 | 0.91 ± 0.02 | <0.001 |
| Fasting glucose, mmol/liter | 4.82 ± 0.09 | 4.89 ± 0.10 | 0.6 |
| 2-hr glucose, mmol/liter* | 5.18 ± 0.43 | 6.46 ± 0.28 | 0.02 |
| Fasting insulin, pmol/liter | 35 ± 2 | 69 ± 10 | 0.01 |
| 2-hr insulin, pmol/liter* | 229 ± 55 | 514 ± 100 | 0.04 |
*Oral glucose tolerance test. For all parameters, the mean and standard error of the mean are shown. For the classification of lean vs. obese, a cut-off in percentage of body fat of 21% and 27% was used in men and women, respectively; this corresponds to a BMI of ≈25 kg/m2.
In addition, we genotyped those 25 subjects and found six heterozygous carriers of the Arg allele of the IRS-1 Gly972Arg polymorphism. We additionally studied four hetero- and one homozygous Arg allele carriers, who were recruited from a larger preexisting database (41). Afterward, a control group of 11 wild-type carriers (Gly/Gly) matched for gender, age, and BMI was selected. The subject characteristics of both groups are given in Table 2.
Experimental Design.
It is well known that neuronal activity of the cerebral cortex measured by MEG/electroencephalography displays daytime variations (26). Because our protocol consisted of a 4- to 5-hr study period, we decided to perform a control experiment with saline infusion to correct for daytime variability. Subjects participated in an insulin and a placebo (saline) experiment in random order on two different days no more than 5 days apart.
Each experimental session began at ≈7.00 a.m. and consisted of a 60-min baseline period and a two-step hyperinsulinemic euglycemic clamp or saline infusion. In both experiments, a dorsal hand vein was cannulated retrogradely for recurrent blood sampling, and a contralateral antecubital vein was cannulated for infusion of insulin/glucose or saline. The blood sampling cannula was preferentially placed in the left hand, and this hand and arm were warmed up with gel heating pads to enable arterialized blood sampling. The two-step hyperinsulinemic-euglycemic clamp consisted of a 90-min low-dose insulin (0.25 milliunit/kg per min) infusion step and a 90-min high-dose insulin (1.0 milliunit/kg per min) infusion step. Both steps were started with an insulin bolus (6.25 milliunits/kg and 17.75 milliunits/kg, respectively). The participants were blind as to whether insulin or saline was infused. A 30-min MEG block was performed at the end of the baseline period and at the end of each experimental step. A similar insulin infusion rate has been shown to produce a doubling of cerebrospinal fluid insulin concentrations in healthy individuals (42).
To maintain blood glucose at baseline levels, a standard hyperinsulinemic euglycemic clamp protocol was followed. Blood was drawn every 5–10 min for determination of blood glucose, and the infusion rate of exogenous glucose was adjusted appropriately to maintain euglycemia. To avoid hypoglycemia, a plasma glucose concentration of 5 mmol/liter was targeted, leaving a buffer for clamp-inherent downward fluctuations without triggering counterregulation. Blood for determination of plasma insulin, as well as cortisol, growth hormone, and epinephrine (data not shown) was sampled at −30 and 0 min and at 60 and 90 min of each step. During the saline experiment, glucose was not infused to avoid secretion of endogenous insulin; this allowed plasma glucose to take the slight physiologic decrease expected during that time of the day. Infusion pumps were located outside the shielded MEG chamber. During the 30-min MEG period, blood was drawn through a fine-bore line from outside of the chamber.
MEG.
We chose MEG parameters that permitted sensitive assessment of both spontaneous cortical activity and stimulated cortical activity (discrimination between two sound qualities, MMN). Auditory MMN of frequency deviation is independent of alertness or attention and is considered to be a robust parameter of preconscious cortical information processing (27, 28).
Magnetoencephalographic signals were recorded with a 151-channel, whole-head MEG system. The subjects were seated in a chair in a magnetically shielded room, and the head was positioned as tightly as possible to the MEG sensors. In addition, the position of the head in relation to the sensors was determined at the beginning and end of each recording session by three head coils, which were attached to the subjects' nasion and left and right periauricular points. If the head movement exceeded 0.5 cm between the start and the end of the recording session, then the measurement was repeated.
Data were recorded in a continuous mode (sampling rate, 625 Hz), starting with eyes open and closed (counterbalanced over sessions and subjects) for 1.5 min each, followed by a standard frequency auditory mismatch experiment. The tones used for the auditory mismatch were 50 ms 1 kHz for the standard and 960 Hz for the deviant in a relation of 80% to 20%. Sixty-decibel tones were presented binaurally. The interstimulus interval was 1,250 ms with a randomization of ±200 ms, resulting in 640 standard and 160 deviant tones. The tones were delivered into the ear by a plastic tube to eliminate electromagnetic interference by headphones. In addition, subjects fixated on a red cross displayed on a screen at a 1-m distance to reduce eye movement artefacts Offline, the recording sessions were separated into two 90-s trials containing the eyes-open and eyes-closed condition and two data sets containing single trials (−100 ms and 500 ms) for standard and deviant tones. After exclusion of trials containing eye movements, the evoked magnetic responses were calculated by stimulus-triggered averaging, and the mismatch field was calculated as the difference between averages to standard and deviant tones (27). For the analysis of the mismatch field, two subjects in the lean group and one subject in the obese group had to be excluded because of recording artefacts during the mismatch task.
For each subject, the magnetic activity was calculated in accordance with standard procedures. For determining an insulin-effect parameter, the difference between the insulin condition and the saline condition was calculated. The power spectrum for the spontaneous activity was analyzed by a statistical mapping procedure. We used an extended approach described in Lutzenberger et al. (43) that corrects for multiple comparisons by randomization. In the current analysis, we detected significant power spectral differences in frequency bands by repeated-measures ANOVA, including all conditions and levels.
For the evaluation of the mismatch field, the root mean square over all MEG channels, as a global activation index, was calculated for a time window, starting at 100 ms and ending at 200 ms after trigger, containing the maximum power of the mismatch field. For statistical analysis, the logarithmically transformed quotient of the root mean square under insulin vs. saline was calculated, resulting in a unitless value.
Genetic Analysis.
The IRS-1 Gly972Arg polymorphism was determined by PCR and subsequent restriction enzyme analysis as described in ref. 41.
Statistical Analysis.
Data from the lean and obese groups were compared using an unpaired Student's t test. Nonnormally distributed variables were logarithmically transformed. Correlations were calculated using least-squares regression analysis. A P value of <0.05 was considered to indicate statistical significance.
For statistical analyses of the MEG experiments, saline experiment data were subtracted from the insulin experiment data. This difference was further analyzed by a repeated-measures ANOVA containing the between-factor (lean and obese) and the repeated-measure factor level (baseline and first and second step of insulin infusion). MEG parameters were calculated using spss 12.0 (SPSS, Chicago) incorporating the Greenhouse–Geisser correction. Otherwise, the software package jmp (SAS Institute, Cary, NC) was used.
Acknowledgments
We thank Anna Teigeler, Heike Luz, Gabi Walker, and Viktor Müller for their superb technical assistance. This study was supported by Deutsche Forschungsgemeinschaft Grant KFO 114 and a Fortüne grant of the University of Tübingen.
Abbreviations
- BMI
body mass index
- IRS
insulin receptor substrate
- MEG
magnetoencephalography
- MMN
mismatch negativity
Footnotes
Conflict of interest statement: No conflicts declared.
References
- 1.Folli F., Bonfanti L., Renard E., Kahn C. R., Merighi A. J. Neurosci. 1994;14:6412–6422. doi: 10.1523/JNEUROSCI.14-11-06412.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Havrankova J., Roth J., Brownstein M. Nature. 1978;272:827–829. doi: 10.1038/272827a0. [DOI] [PubMed] [Google Scholar]
- 3.Hopkins D. F., Williams G. Diabet. Med. 1997;14:1044–1050. doi: 10.1002/(SICI)1096-9136(199712)14:12<1044::AID-DIA508>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 4.Woods S. C., Lotter E. C., McKay L. D., Porte D., Jr. Nature. 1979;282:503–505. doi: 10.1038/282503a0. [DOI] [PubMed] [Google Scholar]
- 5.Schwartz M. W., Bergman R. N., Kahn S. E., Taborsky G. J., Jr., Fisher L. D., Sipols A. J., Woods S. C., Steil G. M., Porte D., Jr. J. Clin. Invest. 1991;88:1272–1281. doi: 10.1172/JCI115431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Porte D., Jr., Seeley R. J., Woods S. C., Baskin D. G., Figlewicz D. P., Schwartz M. W. Diabetologia. 1998;41:863–881. doi: 10.1007/s001250051002. [DOI] [PubMed] [Google Scholar]
- 7.Schwartz M. W., Woods S. C., Porte D., Jr., Seeley R. J., Baskin D. G. Nature. 2000;404:661–671. doi: 10.1038/35007534. [DOI] [PubMed] [Google Scholar]
- 8.Obici S., Zhang B. B., Karkanias G., Rossetti L. Nat. Med. 2002;8:1376–1382. doi: 10.1038/nm1202-798. [DOI] [PubMed] [Google Scholar]
- 9.Bruning J. C., Gautam D., Burks D. J., Gillette J., Schubert M., Orban P. C., Klein R., Krone W., Muller-Wieland D., Kahn C. R. Science. 2000;289:2122–2125. doi: 10.1126/science.289.5487.2122. [DOI] [PubMed] [Google Scholar]
- 10.Biessels G. J., Kamal A., Urban I. J., Spruijt B. M., Erkelens D. W., Gispen W. H. Brain Res. 1998;800:125–135. doi: 10.1016/s0006-8993(98)00510-1. [DOI] [PubMed] [Google Scholar]
- 11.Cranston I., Marsden P., Matyka K., Evans M., Lomas J., Sonksen P., Maisey M., Amiel S. A. J. Cereb. Blood Flow Metab. 1998;18:130–140. doi: 10.1097/00004647-199802000-00002. [DOI] [PubMed] [Google Scholar]
- 12.Eastman R. C., Carson R. E., Gordon M. R., Berg G. W., Lillioja S., Larson S. M., Roth J. J. Clin. Endocrinol. Metab. 1990;71:1602–1610. doi: 10.1210/jcem-71-6-1602. [DOI] [PubMed] [Google Scholar]
- 13.Hasselbalch S. G., Knudsen G. M., Videbaek C., Pinborg L. H., Schmidt J. F., Holm S., Paulson O. B. Diabetes. 1999;48:1915–1921. doi: 10.2337/diabetes.48.10.1915. [DOI] [PubMed] [Google Scholar]
- 14.Benedict C., Hallschmid M., Hatke A., Schultes B., Fehm H. L., Born J., Kern W. Psychoneuroendocrinology. 2004;29:1326–1334. doi: 10.1016/j.psyneuen.2004.04.003. [DOI] [PubMed] [Google Scholar]
- 15.Hallschmid M., Benedict C., Schultes B., Fehm H. L., Born J., Kern W. Diabetes. 2004;53:3024–3029. doi: 10.2337/diabetes.53.11.3024. [DOI] [PubMed] [Google Scholar]
- 16.Hallschmid M., Schultes B., Marshall L., Molle M., Kern W., Bredthauer J., Fehm H. L., Born J. Diabetes. 2004;53:2202–2208. doi: 10.2337/diabetes.53.9.2202. [DOI] [PubMed] [Google Scholar]
- 17.Kern W., Born J., Schreiber H., Fehm H. L. Diabetes. 1999;48:557–563. doi: 10.2337/diabetes.48.3.557. [DOI] [PubMed] [Google Scholar]
- 18.Craft S., Newcomer J., Kanne S., Dagogo-Jack S., Cryer P., Sheline Y., Luby J., Dagogo-Jack A., Alderson A. Neurobiol. Aging. 1996;17:123–130. doi: 10.1016/0197-4580(95)02002-0. [DOI] [PubMed] [Google Scholar]
- 19.Almind K., Inoue G., Pedersen O., Kahn C. R. J. Clin. Invest. 1996;97:2569–2575. doi: 10.1172/JCI118705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McGettrick A. J., Feener E. P., Kahn C. R. J. Biol. Chem. 2005;280:6441–6446. doi: 10.1074/jbc.M412300200. [DOI] [PubMed] [Google Scholar]
- 21.Sesti G., Federici M., Hribal M. L., Lauro D., Sbraccia P., Lauro R. FASEB J. 2001;15:2099–2111. doi: 10.1096/fj.01-0009rev. [DOI] [PubMed] [Google Scholar]
- 22.Baroni M. G., Arca M., Sentinelli F., Buzzetti R., Capici F., Lovari S., Vitale M., Romeo S., Di Mario U. Diabetologia. 2001;44:367–372. doi: 10.1007/s001250051628. [DOI] [PubMed] [Google Scholar]
- 23.Jellema A., Zeegers M. P., Feskens E. J., Dagnelie P. C., Mensink R. P. Diabetologia. 2003;46:990–995. doi: 10.1007/s00125-003-1126-4. [DOI] [PubMed] [Google Scholar]
- 24.Marini M. A., Frontoni S., Mineo D., Bracaglia D., Cardellini M., De Nicolais P., Baroni A., D'Alfonso R., Perna M., Lauro D., et al. J. Clin. Endocrinol. Metab. 2003;88:3368–3371. doi: 10.1210/jc.2002-021716. [DOI] [PubMed] [Google Scholar]
- 25.Clausen J. O., Hansen T., Bjorbaek C., Echwald S. M., Urhammer S. A., Rasmussen S., Andersen C. B., Hansen L., Almind K., Winther K., et al. Lancet. 1995;346:397–402. doi: 10.1016/s0140-6736(95)92779-4. [DOI] [PubMed] [Google Scholar]
- 26.Cummings L., Dane A., Rhodes J., Lynch P., Hughes A. M. Br. J Clin. Pharmacol. 2000;50:21–26. doi: 10.1046/j.1365-2125.2000.00205.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Naatanen R. Int. J. Psychophysiol. 2003;48:179–188. doi: 10.1016/s0167-8760(03)00053-9. [DOI] [PubMed] [Google Scholar]
- 28.Lounasmaa O. V., Hamalainen M., Hari R., Salmelin R. Proc. Natl. Acad. Sci. USA. 1996;93:8809–8815. doi: 10.1073/pnas.93.17.8809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Novitski N., Tervaniemi M., Huotilainen M., Naatanen R. Brain Res. Cogn. Brain Res. 2004;20:26–36. doi: 10.1016/j.cogbrainres.2003.12.011. [DOI] [PubMed] [Google Scholar]
- 30.Tandon O. P., Verma A., Ram B. K. Indian J. Physiol. Pharmacol. 1999;43:383–388. [PubMed] [Google Scholar]
- 31.Vanhanen M., Karhu J., Koivisto K., Paakkonen A., Partanen J., Laakso M., Riekkinen P., Sr. NeuroReport. 1996;7:2767–2771. doi: 10.1097/00001756-199611040-00072. [DOI] [PubMed] [Google Scholar]
- 32.Sauseng P., Klimesch W., Doppelmayr M., Hanslmayr S., Schabus M., Gruber W. R. Neurosci. Lett. 2004;354:123–126. doi: 10.1016/j.neulet.2003.10.002. [DOI] [PubMed] [Google Scholar]
- 33.Oakes T. R., Pizzagalli D. A., Hendrick A. M., Horras K. A., Larson C. L., Abercrombie H. C., Schaefer S. M., Koger J. V., Davidson R. J. Hum. Brain Mapp. 2004;21:257–270. doi: 10.1002/hbm.20004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hassing L. B., Grant M. D., Hofer S. M., Pedersen N. L., Nilsson S. E., Berg S., McClearn G., Johansson B. J. Int. Neuropsychol. Soc. 2004;10:599–607. doi: 10.1017/S1355617704104165. [DOI] [PubMed] [Google Scholar]
- 35.Carro E., Torres-Aleman I. Eur. J. Pharmacol. 2004;490:127–133. doi: 10.1016/j.ejphar.2004.02.050. [DOI] [PubMed] [Google Scholar]
- 36.Hennige A. M., Sartorius T., Tschritter O., Preissl H., Fritsche A., Ruth P., Häring H. U. Diabetologia. 2006;49:1274–1282. doi: 10.1007/s00125-006-0192-9. [DOI] [PubMed] [Google Scholar]
- 37.Duckrow R. B. Brain Res. 1988;462:363–366. doi: 10.1016/0006-8993(88)90566-5. [DOI] [PubMed] [Google Scholar]
- 38.Park L., Anrather J., Zhou P., Frys K., Wang G., Iadecola C. Arterioscler. Thromb. Vasc. Biol. 2004;24:1860–1865. doi: 10.1161/01.ATV.0000142446.75898.44. [DOI] [PubMed] [Google Scholar]
- 39.Bingham E. M., Hopkins D., Smith D., Pernet A., Hallett W., Reed L., Marsden P. K., Amiel S. A. Diabetes. 2002;51:3384–3390. doi: 10.2337/diabetes.51.12.3384. [DOI] [PubMed] [Google Scholar]
- 40.Baura G. D., Foster D. M., Porte D., Jr., Kahn S. E., Bergman R. N., Cobelli C., Schwartz M. W. J. Clin. Invest. 1993;92:1824–1830. doi: 10.1172/JCI116773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stumvoll M., Fritsche A., Volk A., Stefan N., Madaus A., Maerker E., Teigeler A., Koch M., Machicao F., Häring H. Diabetes. 2001;50:882–885. doi: 10.2337/diabetes.50.4.882. [DOI] [PubMed] [Google Scholar]
- 42.Watson G. S., Peskind E. R., Asthana S., Purganan K., Wait C., Chapman D., Schwartz M. W., Plymate S., Craft S. Neurology. 2003;60:1899–1903. doi: 10.1212/01.wnl.0000065916.25128.25. [DOI] [PubMed] [Google Scholar]
- 43.Lutzenberger W., Ripper B., Busse L., Birbaumer N., Kaiser J. J. Neurosci. 2002;22:5630–5638. doi: 10.1523/JNEUROSCI.22-13-05630.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]