Abstract
In vertebrates, the endocannabinoid signaling pathway is an important lipid regulatory pathway that modulates a variety of physiological and behavioral processes. N-Acylethanolamines (NAEs) comprise a group of fatty acid derivatives that function within this pathway, and their signaling activity is terminated by an enzyme called fatty acid amide hydrolase (FAAH), which hydrolyzes NAEs to ethanolamine and their corresponding free fatty acids. Bioinformatic approaches led to the identification of plant homologues of FAAH that are capable of hydrolyzing NAEs in vitro. To better understand the role of NAEs in plants, we identified T-DNA knockouts to Arabidopsis FAAH (AtFAAH; At5g64440) and generated plants overexpressing AtFAAH. Here we show that seeds of AtFAAH knockouts had elevated levels of endogenous NAEs, and seedling growth was hypersensitive to exogenously applied NAE. On the other hand, seeds and seedlings of AtFAAH overexpressors had lower endogenous NAE content, and seedlings were less sensitive to exogenous NAE. Moreover, AtFAAH overexpressors displayed enhanced seedling growth and increased cell size. AtFAAH expression and FAAH catalytic activity increased during seed germination and seedling growth, consistent with the timing of NAE depletion during seedling establishment. Collectively, our results show that AtFAAH is one, but not the only, modulator of endogenous NAE levels in plants, and that NAE depletion likely participates in the regulation of plant growth.
Keywords: endocannabinoids, lipids, seedling growth, signaling
The discovery of membrane receptors (cannabinoid receptors) in mammalian brain tissue that bind to marijuana’s principal psychoactive compound, Δ9-tetrahydrocannabinol, led to the establishment of the endocannabinoid signaling pathway as a key regulator of important neurological processes in animals (1). Central to endocannabinoid signaling are the N-acylethanolamines (NAEs), a group of fatty acids with amide linkages to ethanolamine. N-arachidonoylethanolamine, or anandamide (NAE20:4), an NAE type present at low concentrations in mammalian tissues, is an endogenous ligand of cannabinoid (CB) receptors (2). Binding of anandamide to CB receptors triggers a series of events that facilitate neuronal signaling. Although anandamide is the most widely studied NAE in animals, other NAE types have emerged as regulators of important physiological processes such as embryo development, cell proliferation, immune responses, and apoptosis (1).
There is accumulating evidence that plants also use NAEs to regulate important physiological processes. This notion is supported by the identification of NAEs in a variety of plant tissues (3, 4) and the observation that the levels of NAEs in plants, as in mammals, appear to change quite dramatically under certain growth and environmental conditions (5). For instance, NAEs are elevated in desiccated seeds of a variety of plant species (6), but, during imbibition and germination, NAE levels drop significantly and remain at low concentrations during subsequent seedling growth (7, 8). These observations suggest that the rapid metabolism of NAEs during seed germination might be necessary for normal seedling development. In fact, exogenous application of N-lauroylethanolamine (NAE12:0), a naturally occurring plant NAE, inhibits seedling growth, alters cell shape, and influences cytoskeletal dynamics in Arabidopsis (9, 10).
The likelihood that NAEs play a role in plant physiological processes is strongly supported by the fact that the enzymatic machinery for the degradation of NAE is conserved between plants and animals. For example, an enzyme that rapidly hydrolyzes NAEs into ethanolamine and their corresponding free fatty acids has been cloned from mammals. This enzyme, called fatty acid amide hydrolase (FAAH), belongs to a large group of proteins containing a conserved amidase signature sequence (11, 12). The cloning of mammalian FAAH has provided a powerful system by which to investigate the physiological function of NAEs. For instance, the targeted disruption of FAAH in mice resulted in hypersensitivity to exogenous anandamide and a 10-fold elevation of endogenous brain anandamide levels. FAAH knockout mice also displayed physiological abnormalities that were consistent with disrupted endocannabinoid signaling, such as reduced sensation to pain (13). These studies have provided evidence that NAE signal termination in mammals is facilitated by FAAH, and that the activity of FAAH is an important factor regulating endocannabinoid signaling.
We recently reported the molecular identification of a functional homologue of FAAH in Arabidopsis thaliana that converts a wide range of NAEs to their corresponding free fatty acids and ethanolamine (14). Functional homologues of the Arabidopsis FAAH (AtFAAH) also were identified in Oryza sativa and Medicago truncatula, supporting a common mechanism for the regulation of NAE hydrolysis in diverse plant species (15). To begin to understand the in vivo role of NAEs in plants, we generated Arabidopsis plants with altered AtFAAH expression and analyzed their response to an NAE type that we have previously shown to induce strong morphological effects on seedlings (9, 10). Here, we demonstrate that the manipulation of AtFAAH activity alters the physiological responses of Arabidopsis seedlings to exogenously applied NAEs. The enhanced seedling growth of AtFAAH overexpressors, the hypersensitivity of AtFAAH knockouts to exogenous NAE, and the increased expression and enzymatic activity of AtFAAH, which coincides with NAE depletion during seed germination, all are consistent with the notion that these fatty acid amides may be negative regulators of seedling growth. We propose that FAAH modulates endogenous NAE levels in plants and functions as a metabolic correlate to the endocannabinoid signaling enzyme found in animals.
Results
FAAH Is Expressed in Different Organs, and Its Expression and Activity Are Consistent with the Timing of NAE Depletion During Seed Germination and Seedling Growth.
Expression of the AtFAAH gene was evaluated by quantitative RT-PCR (Fig. 1). AtFAAH expression was detected in various organs and at different developmental stages. Transcript levels were plotted relative to those in inflorescence stems (lowest transcript levels; Fig. 1A). Among the tissues of mature plants, transcript levels were highest in siliques (18-fold higher than those measured in the stem). AtFAAH transcript levels in desiccated seeds were 2.5-fold higher than those in stems, whereas FAAH transcript levels were 4- and 19-fold higher in imbibed seeds and 4-d-old seedlings, respectively (Fig. 1A). The higher transcript levels in imbibed seeds and seedlings were consistent with the notion that NAE metabolism is activated during seed germination and seedling development. The generally constitutive nature of endogenous AtFAAH expression was consistent with information in publicly available microarray databases (ref. 16; www.weigelworld.org/resources/microarray/AtGenExpress) as compiled for At5g64440 by Beisson et al. (ref. 17; www.plantbiology.msu.edu/lipids/genesurvey/FAAH.htm).
Fig. 1.
FAAH expression in Arabidopsis. (A) AtFAAH mRNA transcript levels quantified in seeds, seedlings, and different organs of 6-week-old Arabidopsis plants by quantitative real-time RT-PCR. ACT8 was used to normalize AtFAAH expression levels and plotted relative to transcript levels in inflorescence stems. AtFAAH was expressed in all plant organs with highest levels quantified in 4-d-old seedlings and siliques of 6-week-old plants. Data points represent mean ± SD of triplicates of an experiment. (B) AtFAAH::GUS is expressed strongly in embryos 24–48 h after imbibition and in 4-d-old seedlings. The depletion of total NAEs in germinating seeds (C) correlated strongly with increased FAAH enzyme activity toward NAE12:0 and NAE18:2 (D).
To visualize spatial patterns of AtFAAH expression within different plant organs, we cloned a 1.8-kb DNA fragment upstream of the AtFAAH coding sequence. This fragment included a 1.3-kb promoter region, 5′-UTR, and the first intron of the AtFAAH was fused to a β-glucuronidase (GUS) reporter gene. Transgenic plants harboring the AtFAAH::GUS reporter construct were generated and their GUS expression patterns examined. Consistent with our RT-PCR analysis, weak GUS expression was detected in embryos of seeds imbibed for 30 min, whereas strong GUS expression was observed in 4-d-old seedlings (Fig. 1B). AtFAAH::GUS expression in mature plant organs also was consistent with the RT-PCR results. Only weak GUS expression was observed in inflorescence stems, leaves, and flowers (data not shown).
Importantly, the pattern of AtFAAH::GUS expression in seeds and seedlings mirrored the depletion of NAEs during seed germination and seedling growth. In desiccated Arabidopsis seeds, total NAE content was ≈2,000 ng per gram, and these levels declined significantly 24–192 h after sowing (Fig. 1C). Increased AtFAAH::GUS expression in dissected embryos at 24 and 48 h after imbibition and 4-d-old seedlings was consistent with the catabolism of total NAEs during germination and early postgerminative seedling growth (Fig. 1B). Moreover, the depletion of total seed NAEs during germination and enhanced AtFAAH::GUS expression was accompanied by increased FAAH enzyme activity (Fig. 1D).
Manipulation of Arabidopsis FAAH Gene Expression.
We obtained mutants for AtFAAH (At5g64440) from the SALK T-DNA mutant collection. Two T-DNA insertional mutants SALK_095108 and SALK_118043 were identified with a T-DNA insertion in the 13th intron and the 17th exon, respectively (Fig. 6, which is published as supporting information on the PNAS web site). Overexpressing FAAH lines were generated by placing the AtFAAH cDNA under the control of the cauliflower mosaic virus (CaMV) 35S promoter (35S::AtFAAH; Fig. 6). Several independent overexpressing lines were generated, and Southern blot analysis identified lines with single copy of the transgene (data not shown). Three independent T3 FAAH-overexpressing lines (FAAHoe-2, FAAHoe-7, and FAAHoe-11) were chosen for further studies. Semiquantitative RT-PCR analysis revealed that the two SALK knockout lines had no detectable FAAH transcripts, whereas overexpressors had elevated FAAH transcript levels compared with wild type (Fig. 6).
FAAH-Impaired Arabidopsis Seedlings Exhibit Reduced NAE Amidohydrolase Activity and Enhanced Sensitivity to Exogenous NAE.
We next analyzed the NAE hydrolyzing activity of the AtFAAH knockouts to determine whether the lack of transcript caused a corresponding reduction in enzyme activity. Indeed, cell-free homogenates and microsomes isolated from AtFAAH knockouts had reduced NAE hydrolytic activity in vitro, compared with wild type when NAE18:2, NAE12:0 (Fig. 2A), or NAE16:0 (data not shown) was used as a substrate, suggesting that the endogenous capacity for general NAE catabolism had been altered in these knockout lines.
Fig. 2.
Characterization of NAE amidohydrolase activity and NAE sensitivity of AtFAAH knockouts. (A) Reduced NAE amidohydrolase activity in microsomes of knockouts is indicated by the absence of free fatty acid (FFA) peak. The amount of FFA formation was determined by incubating synthetic NAE18:2 and NAE12:0 with microsomes from seedlings. (B) NAE12:0 induced a dose-dependent reduction in seedling development in Arabidopsis wild type as indicated by reduced cotyledon area. The effects of NAE12:0 on Arabidopsis cotyledon area was more pronounced in AtFAAH knockouts. (C) Wild-type seedlings are able to recover from exogenous NAE12:0 2 weeks after germination, whereas AtFAAH knockouts remain severely stunted.
Despite the lower NAE amidohydrolase activity in these Arabidopsis plants, there were no gross changes in overall morphology, although seedling growth and rosette diameter were somewhat reduced in one line (Table 1, which is published as supporting information on the PNAS web site). To determine whether Arabidopsis lacking endogenous FAAH activity displayed shifts in sensitivity toward exogenous NAEs, we germinated AtFAAH knockouts in a range of NAE12:0 concentrations. We showed previously that exogenous NAE12:0 resulted in severe inhibitory effects on Arabidopsis seedling growth, manifested as reduced cotyledon expansion and shorter hypocotyls and primary roots (9, 10). Therefore, the distinct morphological effects induced by NAE12:0 provided us with a clear biological assay by which to test in vivo AtFAAH function and sensitivity to NAE. Wild type and AtFAAH knockouts germinated on media supplemented with NAE12:0 exhibited various seedling growth defects. In comparison with wild type, the effects of NAE12:0 on the seedling morphology of AtFAAH knockouts were more severe as they displayed a stronger dose-dependent reduction of cotyledon area (Fig. 2B). The differences between wild type and AtFAAH knockouts were most pronounced at NAE12:0 concentrations between 20 and 30 μM. Upon prolonged exposure (after 2 weeks) to 30 μM NAE12:0, wild-type seedlings normally recovered (9), most likely because of endogenous AtFAAH activity. However, both AtFAAH knockout lines were unable to resume growth on NAE12:0 and remained severely stunted (Fig. 2C). Collectively, these results confirm that the gene, At5g64440, encodes a functional NAE amidohydrolase and clearly indicate that disruption of this gene leads to predictable enhanced sensitivity toward exogenous NAE12:0.
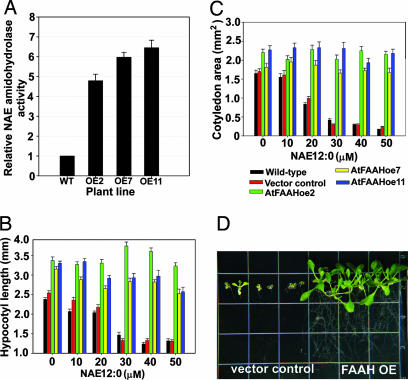
Overexpression of FAAH Leads to Increased NAE Amidohydrolase Activity, Reduced Sensitivity to Exogenous NAE, and Enhanced Seedling/Plant Growth.
Cell-free homogenates from AtFAAH overexpressors had up to 6.5-fold higher levels of NAE amidohydrolase activity compared with wild type (Fig. 3A). We anticipated that NAE12:0 would not inhibit growth of the AtFAAH overexpressors to the same degree as the wild type or knockouts. Indeed, seedlings of AtFAAH overexpressors displayed less sensitivity toward exogenous NAE12:0 compared with the “empty-vector” controls or wild-type seedlings. Notably, cotyledon and hypocotyl expansion of the overexpressors was not significantly inhibited, despite the increasing levels of exogenous NAE12:0 (Fig. 3 B and C). The most obvious difference between AtFAAH overexpressors and vector controls in terms of their response to exogenous NAE was during extended exposures (>18 days) to high levels of NAE12:0 (up to 500 μM). Under these conditions, empty vector control seedlings were severely stunted, whereas AtFAAH overexpressors proceeded to develop significantly larger organs (Fig. 3D).
Fig. 3.
Characterization of NAE amidohydrolase activity and NAE sensitivity of AtFAAH overexpressors. (A) Increased NAE amidohydrolase activity in microsomes of three AtFAAH-overexpressing lines. The amount of free fatty acid formation was determined by incubating synthetic NAE18:2, NAE16:0, and NAE12:0 with microsomes from seedlings. Three independent AtFAAH overexpressors were able to sustain hypocotyl (B) and cotyledon (C) expansion despite elevated levels of exogenous NAE12:0. (D) Extended exposure to 500 μM NAE12:0 is strongly inhibitory to vector control and wild-type seedlings. AtFAAH overexpressors, on the other hand, display robust growth despite the high levels and extended exposure to exogenous NAE12:0.
Interestingly, when compared with wild type and empty-vector controls, AtFAAH overexpressors exhibited enhanced growth at different stages of development (Table 1). Seedlings of AtFAAH overexpressors had generally longer primary roots and hypocotyls, larger cotyledon areas (Fig. 4 A and B; Table 1), and greater seedling fresh weight (Fig. 7, which is published as supporting information on the PNAS web site). In addition to an overall increase in seedling growth, AtFAAH overexpressors displayed robust vegetative growth under short or long day conditions (Fig. 4C; Table 1) and bolted earlier (Fig. 4D; Table 1). The average heights of the inflorescences of AtFAAH overexpressors were substantially greater compared with wild type (Table 1). Because the increased cotyledon area was a distinct phenotype of the AtFAAH overexpressors (Fig. 4B), we measured average epidermal cell size from cotyledons of 11-d-old AtFAAH overexpressors and vector controls using the membrane dye FM 4–64 to clearly mark the outline of the individual epidermal cells. The average epidermal cell size from AtFAAH overexpressors was ≈25% larger than that of the vector controls (Fig. 4E). This was due to overexpressors having more cells with a larger area than cells from cotyledons of control seedlings (Fig. 4F). In summary, overexpression of AtFAAH appeared to have a positive impact on both growth and overall organ size, suggesting that an increase in capacity for NAE catabolism promoted plant growth.
Fig. 4.
Enhanced growth of AtFAAH overexpressors. (A) Representative images of vector controls and AtFAAH-overexpressing seedlings 11 days after germination. Primary root and hypocotyl length is longer (A, arrows) and cotyledon area is larger (B) in the AtFAAH overexpressors. One-month-old plants of the AtFAAH overexpressors are generally larger than vector controls when grown under short days (C) and generally bolt faster (D). The larger cotyledon area in AtFAAH overexpressors is due to larger average epidermal cell size (E) and increased number of epidermal cells with a larger cell area (F). (Scale bar in C, 1 mm.)
FAAH Knockouts and Overexpressors Possess Altered Endogenous NAE Levels.
In plants, NAEs are readily quantifiable in desiccated seeds, but their levels change dramatically during germination and seedling growth (Fig. 1C; refs. 6 and 7). Therefore, we first quantified NAEs in desiccated seeds of AtFAAH-altered plants, because the quiescent nature of seeds provided an opportunity to capture the “resting” levels of NAEs. We predicted that plants with altered AtFAAH expression should have modified levels of NAEs in their desiccated seeds. Indeed, total NAE content was 30% higher in seeds of the AtFAAH knockout line SALK_095108 (Fig. 5A), and most of this increase was attributable to higher levels of the major unsaturated 18C NAE types (NAE18:1, NAE18:2, and NAE18:3) and one saturated NAE type (NAE16:0) compared with wild type (Fig. 5B). There was little difference in the minor saturated NAE species (NAE12:0, NAE14:0, and NAE18:0) between knockout and wild-type seeds (Fig. 5B Inset). On the other hand, AtFAAH overexpressors exhibited consistently lower levels of total NAE content in seeds compared with wild type. For example, seeds of FAAHoe-11 had about one-half the total NAE content measured in wild-type seeds (Fig. 5A). These substantial differences in total NAE content were attributable to the major NAE types, NAE18:1, NAE18:2, NAE18:3, and NAE16:0 (Fig. 5B), whereas the minor saturated NAE types showed little change (Fig. 5B Inset).
Fig. 5.
Comparison of NAE profiles in desiccated Arabidopsis seeds and seedlings of wild type, At5g64440 knockout (SALK_095108), and AtFAAH overexpressors (OE11). NAE types were quantified by isotope-dilution mass spectrometry and summed for total content (A) or plotted individually (B and C). Values represent mean + SD of three to six independent extractions from seeds and seedlings of plants that were grown and harvested at the same time (within a 3-month period). Seeds were stored under identical conditions, whereas seedlings were grown under liquid culture for 8 days before NAE quantification. P values were obtained by Student’s t test.
Endogenous NAE levels were also quantified in 8-d-old seedlings, because the differences in sensitivity to exogenous NAE and enhanced growth of AtFAAH overexpressors become clearly apparent at this stage (Figs. 3 and 4). All genotypes showed a significant reduction in endogenous NAE levels during germination, as evident from the lower total NAE content of 8-d-old seedlings (Fig. 5A). Differences among genotypes were not as obvious in seedlings as in desiccated seeds. Seedlings of AtFAAH overexpressors had ≈15% lower total NAE content compared to wild type, whereas knockout seedlings had total NAE levels that were ≈10% higher than wild type (Fig. 5A). However, on closer inspection of NAE profiles, a clear difference among overexpressors, knockouts, and wild type was evident (Fig. 5C). As in seeds, the difference was predominantly in levels of 18C polyunsaturated NAEs. NAE18:3 and NAE18:2 levels in overexpressors were 40–60% lower than wild type or knockouts. The levels of other NAE types were relatively similar among genotypes. These results suggested that AtFAAH overexpression selectively lowered polyunsaturated NAE levels in transgenic seeds and seedlings, and that differences in seedling growth were likely associated with altered endogenous NAE18:2 (and NAE18:3) levels. Earlier studies indicated that NAE16:0 had no effect on seedling growth (9). Here, we confirmed that, like NAE12:0, exogenous NAE18:2 indeed reduced seedling growth in a potent dose-dependent manner (Fig. 8, which is published as supporting information on the PNAS web site). NAE18:2 reduced growth of wild-type and knockout seedlings substantially; and AtFAAH overexpressors, as might be predicted, displayed tolerance to the inhibitory effects of exogenous NAE18:2 (Fig. 8).
Discussion
The widespread occurrence of NAEs in plants and the manner by which their levels are regulated during seed germination and postgerminative seedling growth (7) indicate that these fatty acid amides, which are known to have diverse physiological functions in mammals, might also have important roles in plant development (5). However, there has been little information on the function of these fatty acid amides in plant physiology. In animals, NAE function is terminated by the hydrolysis to free fatty acid and ethanolamine, and this reaction is facilitated by FAAH (12). The cloning of the mammalian FAAH led to a series of important studies on the in vivo consequences of reduced FAAH activity and constitutive elevation of endogenous NAEs in animal physiology (13, 18). For example, the targeted disruption of FAAH in mice resulted in highly exaggerated behavioral responses to anandamide, which typically exerts only transient and weak effects when administered exogenously (13). Here, the consequences of disrupted FAAH in Arabidopsis showed a remarkable similarity to the scenario in mice. Two knockout lines to the AtFAAH gene lacked significant NAE hydrolase activity, and their seedlings displayed enhanced sensitivity to exogenous NAE12:0. On the other hand, seedlings constitutively overexpressing AtFAAH exhibited elevated NAE hydrolase activity, which was coupled with the ability of these seedlings to grow in exogenous levels of NAE12:0 that would typically inhibit wild-type seedling development (9, 10). Moreover, AtFAAH knockouts had ≈30% increase in total seed NAEs, whereas AtFAAH-overexpressing lines showed a 20–50% reduction in total seed NAE. In seedlings, evidence for FAAH-mediated hydrolysis is more complex and appears to be combined with an alternative pathway for the depletion of NAEs. Nonetheless, our results provide evidence that AtFAAH, like its mammalian counterpart, degrades NAEs in vivo.
Several key observations suggest that NAEs might function as negative regulators of processes associated with cell expansion and seedling growth. First, seed germination and seedling establishment depend upon synchronized cell division and expansion, and AtFAAH expression and NAE hydrolase activity increased during Arabidopsis seed germination and early seedling development concomitant with the depletion of endogenous NAEs (Fig. 1). Second, elevated levels of exogenous NAE12:0 profoundly disrupted normal cell expansion in seedlings in a dose-dependent and selective manner (9, 10). Here we showed that NAE18:2, which is the NAE type that exhibits the most dramatic decline during seed germination, can also inhibit seedling growth (Fig. 8). Third, constitutive overexpression of AtFAAH led to plants with reduced NAE content in their seeds and seedlings (Fig. 5), especially in terms of polyunsaturated 18C NAEs. Fourth, the reduction in seed and seedling polyunsaturated NAE content was accompanied by accelerated seedling growth and organs that were significantly larger in size (Figs. 3 and 4). This accelerated growth phenotype was observed in multiple overexpressing lines and persisted at later stages of plant development (Fig. 4; Table 1). Based on the experimental evidence, we conclude that the metabolism of NAE is associated with cell expansion during early seedling growth and possibly overall plant growth. However, the precise mechanism underlying NAE metabolism and plant growth may involve a more complex signaling network, because there are multiple types of NAEs in plants, and these may give rise to alternative products (such as NAE oxylipins; refs. 8 and 19), and/or FAAH may act on alternative substrates (e.g., sn-2 monoacylglycerols in mammals; ref. 20). Future work will be necessary to determine the precise mechanism of FAAH action in plants, but it is likely that this enzymatic pathway participates in the regulation of seedling growth by modulating endogenous NAE levels.
An analysis of levels of individual NAE species revealed that the principal NAE types that were lower in seeds and seedlings of AtFAAH overexpressors were the long-chain 18C unsaturated species (Fig. 5C). NAE18:2, which like NAE12:0 induces strong inhibitory effects on seedling growth when applied exogenously (Fig. 8), was >50% lower in seedlings of AtFAAH overexpressors (Fig. 5C). The reduced NAE18:2 levels indicate that this NAE type may be more relevant to the enhanced seedling growth phenotype of AtFAAH overexpressors, and removal of the long-chain unsaturated NAEs could in part be responsible for the larger size of AtFAAH overexpressors. Indeed, AtFAAH overexpressors start out with less overall NAE in their seeds and have lower levels of unsaturated 18C NAEs during seedling development, suggesting that the ability to maintain a reduced 18C NAE concentration may be responsible for accelerated growth relative to wild type. However, it is notable that AtFAAH overexpressors did not display the same level of tolerance to exogenous NAE18:2 as they did to NAE12:0 (Fig. 8). The basis for these differences in sensitivity is not clear, but it is possible that breakdown/oxidation products for each NAE type (e.g., lauric acid for NAE12:0 and linoleic acid or NAE-oxylipins for NAE18:2) might somehow influence the overall growth response of seedlings to exogenous NAE application and to changing endogenous NAE levels resulting from AtFAAH overexpression. Although additional studies will be required to clarify these issues, our AtFAAH-altered plants, in combination with metabolite measurements and enzyme activity assays, should allow an in-depth analysis of how specific NAE types influence various aspects of plant development.
Despite the reduced NAE amidohydrolase activity in AtFAAH knockouts, we did not observe dramatic growth phenotypes in these lines. Because elevation of NAEs by exogenous application showed a dramatic retardation of seedling development (refs. 9 and 10; Figs. 2 and 8), we expected that the AtFAAH knockouts would exhibit stunted seedling growth, presumably because of the accumulation of endogenous NAEs. Although total endogenous NAEs were higher in seeds of AtFAAH knockouts relative to the wild type (Fig. 5 A and B), it was surprising that AtFAAH knockout seedlings appeared capable of depleting seed NAEs to levels similar to wild type (Fig. 5C), particularly because no detectable NAE hydrolase activity was measured in seedling homogenates by using NAE12:0 or NAE18:2 as substrates (Fig. 2). However, this does provide an explanation as to why the growth of knockout seedlings was similar to that of wild type. It also suggests that an alternative pathway exists for NAE depletion in seedlings. Interestingly, a competing oxidation pathway was identified in cotton seedlings that operated by 13-lipoxygenase (and allene oxide synthase) to produce NAE oxylipins (8), and it is possible that flux through this pathway is considerable during seedling growth. It is also possible that a second unidentified amidase is responsible for NAE hydrolysis in knockouts. In this regard, it is worth mentioning that an acid amidase has been identified in vertebrates capable of hydrolyzing NAEs (21), and perhaps a similar mechanism operates in AtFAAH knockout plants. In any case, taken together, our results demonstrate that AtFAAH expression influences NAE content in planta, but they also point to additional factors that determine the overall NAE profiles during seed germination and seedling growth. Additional studies will be needed to understand the interaction between NAE oxidation by lipoxygenase and NAE hydrolysis by FAAH in regulating NAE flux in seedlings, and the availability of AtFAAH knockouts and overexpressors will help to tease apart the complexities of NAE metabolism and the regulation of seedling growth.
Materials and Methods
Plant Material and Growth Assays.
Two T-DNA insertion mutants were identified from the SALK collection (SALK_095108 and SALK_118043; ref. 22) and provided by the Arabidopsis Biological Resource Stock Center (Ohio State University, Columbus, OH). The precise location and orientation of the T-DNA inserts were confirmed by DNA sequencing of PCR products amplified with T-DNA and gene-specific primers. To constitutively express AtFAAH, an AtFAAH cDNA (without stop codon) was PCR-amplified from a previously reported expression construct in pTrcHis2 (16) using primers designed for cloning at the SalI/EcoRI site. To generate the 35S::AtFAAH construct, the AtFAAH cDNA was placed behind a cauliflower mosaic virus 35S promoter that had been previously introduced into a pCAMBIA-1390 vector (23). To generate the AtFAAH::GUS reporter construct, a 1,834-bp DNA fragment that included a 1,364-bp putative promoter region, the 5′-UTR and the first intron of AtFAAH was PCR-amplified from Arabidopsis genomic DNA. The PCR product was cloned into a Gateway entry vector (pCR8/GW/TOPO TA vector; Invitrogen, Carlsbad, CA) and then introduced into the destination vector, pMDC165 (24). Transgenic Arabidopsis was generated by the floral-dip method with Agrobacterium tumefaciens harboring the specific construct.
Seeds of AtFAAH knockouts, AtFAAH overexpressors, wild type, and empty vector controls were planted and grown on different concentrations of NAE12:0, as described (9). Seven days after planting, images of the hypocotyls, cotyledons, and primary roots were captured by using a Nikon DMX 1200 digital camera coupled to a Nikon SMZ 1500 stereomicroscope (Melville, NY). In another set of experiments, Arabidopsis seedlings were germinated in liquid culture and after 3 days, NAE18:2 was added to the medium at a final concentration of 20–40 μM. Hypocotyl length, primary root length, and cotyledon area were measured by using Metamorph 4.6 (Universal Imaging, Downington, PA). Statistical analyses were conducted by using Sigma Plot, ver. 6.1, software (SPSS Inc., Chicago, IL).
AtFAAH Expression Analysis.
Total RNA was isolated from different organs of the plants, seeds, imbibed seeds, and seedlings, as described in ref. 25. Real-time RT-PCR was performed in a Smart Cycler II (Cepheid, Sunnyvalle, CA) instrument with a real-time one-step RNA PCR assay kit (Takara Bio, Tokyo, Japan) using SYBR green I dye for quantification of PCR products. ACT8 was used as the internal reference (26) for quantifying relative AtFAAH transcript levels. The primers used for quantification of mRNAs of internal standard (ACT8) and the gene of interest (AtFAAH) were: ACT8 forward (F), 5′-GTTAAGGCTGGATTCGCTGG-3′; ACT8 reverse (R), 5′-GTTAAGAGGAGCCTCGGTAAG-3′; AtFAAH-F, 5′-CCATCTCAAGAACCGGAGCATG-3′; and AtFAAH-R, 5′-GGTGTTGGAGGCTTGTCATAGC-3′. The primers spanned one intron of the genomic sequence, and melting curve analysis and agarose gel electrophoresis were used to optimize PCR conditions and verify results. The comparative cycle threshold (CT) method was used to quantify the relative transcript levels of AtFAAH in different plant parts and stages (27).
Preparation of Microsomes and Analysis of FAAH Activity.
Microsomal fractions were prepared by differential centrifugation as described (8). Protein concentrations in cell fractions were estimated by using BSA as standard according to Bradford (28). FAAH activities were measured with equal amounts (400 μg) of microsomal proteins isolated from 4- to 8-day-old seedlings using [1-14C]NAE12:0 or [1-14C]NAE18:2 as substrates. Enzyme reactions were carried out, and lipid products were extracted as described (8). FAAH-specific activity was quantified by radiometric scanning [Bioscan (Washington, DC) system 200 imaging scanner] of lipid-soluble enzyme reaction products separated by thin-layer chromatography (8).
Chemicals.
[1-14C] linoleic acid (53 mCi·mmol−1 in ethanol; 1Ci = 37 GBq) was purchased from PerkinElmer Life Sciences (Boston, MA), and [1-14C] lauric acid (53 mCi·mmol−1 in ethanol) was from Amersham Pharmacia Biosciences (Piscataway, NJ). Specific types of NAEs were synthesized from respective radiolabeled free fatty acids as described (8). The purity of the NAE substrates was >99.5%.
Quantification of NAE Seeds and Seedlings.
NAE quantification was performed by isotope dilution mass spectrometry from extracts of desiccated seeds and 8-d-old seedlings as described (6). One hundred milligrams of desiccated Arabidopsis seeds (harvested from plants grown at the same time under identical conditions) and seedlings grown in liquid culture were crushed in a ground-glass tissue homogenizer in the presence of hot 2-propanol (70°C) to inactivate endogenous phospholipases (7). A standard mix of deuterated NAEs (50 ng each; see ref. 6) was added for quantification purposes. Total lipids were extracted into chloroform, filtered, and fractionated by normal-phase HPLC with a linear gradient of 2-propanol in hexane, as described (7). Endogenous NAEs were quantified against the internal deuterated standards as tetramethylsilane–ether derivatives by GC-MS (6).
Supplementary Material
Acknowledgments
We thank Mr. Matthew Cotter and Ms. Gia George for assistance with Arabidopsis growth measurements. This work was supported by U.S. Department of Energy Grant DE-FG02–05ER15647 (to K.D.C. and E.B.B.).
Abbreviations
- FAAH
fatty acid amide hydrolase
- AtFAAH
Arabidopsis thaliana FAAH
- NAE
N-acylethanolamine
- GUS
β-glucuronidase.
Footnotes
Conflict of interest statement: No conflicts declared.
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.De Petrocellis L., Cascio M. G., Di Marzo V. Br. J. Pharmacol. 2004;141:765–774. doi: 10.1038/sj.bjp.0705666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Devane W. A., Hanus L., Breuer A., Pertwee R. G., Stevenson L. A., Griffin G., Gibson D., Mandelbaum A., Etinger A., Mechoulam R. Science. 1992;258:1946–1949. doi: 10.1126/science.1470919. [DOI] [PubMed] [Google Scholar]
- 3.Chapman K. D. Chem. Phys. Lipids. 2000;108:221–230. doi: 10.1016/s0009-3084(00)00198-5. [DOI] [PubMed] [Google Scholar]
- 4.Chapman K. D. Prog. Lipid Res. 2004;43:302–327. doi: 10.1016/j.plipres.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 5.Blancaflor E. B., Chapman K. D. In: Communication in Plants–Neuronal Aspects of Plant Life. Baluška F., Mancuso S., Volkmann D., editors. Heidelberg, Germany: Springer; 2006. pp. 205–219. [Google Scholar]
- 6.Venables B. J., Waggoner C. A., Chapman K. D. Phytochemistry. 2005;66:1913–1918. doi: 10.1016/j.phytochem.2005.06.014. [DOI] [PubMed] [Google Scholar]
- 7.Chapman K. D., Venables B., Blair R., Bettinger C. Plant Physiol. 1999;120:1157–1164. doi: 10.1104/pp.120.4.1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shrestha R., Noordimeer M., van der Stelt M., Veldink G., Chapman K. D. Plant Physiol. 2002;130:391–401. doi: 10.1104/pp.004689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Blancaflor E. B., Hou G., Chapman K. D. Planta. 2003;217:206–217. doi: 10.1007/s00425-003-0985-8. [DOI] [PubMed] [Google Scholar]
- 10.Motes C. M., Pechter P., Yoo C.-M., Wang Y.-S., Chapman K. D., Blancaflor E. B. Protoplasma. 2005;226:109–123. doi: 10.1007/s00709-005-0124-4. [DOI] [PubMed] [Google Scholar]
- 11.Cravatt B. F., Giang D. K., Mayfield S. P., Boger D. L., Lerner R. A., Gilula N. B. Nature. 1996;384:83–87. doi: 10.1038/384083a0. [DOI] [PubMed] [Google Scholar]
- 12.McKinney M. K., Cravatt B. F. Annu. Rev. Biochem. 2005;74:411–432. doi: 10.1146/annurev.biochem.74.082803.133450. [DOI] [PubMed] [Google Scholar]
- 13.Cravatt B. F., Demarest K., Patricelli M. P., Bracey M. H., Giang D. K., Martin B. R., Lichtman A. H. Proc. Natl. Acad. Sci. USA. 2001;98:9371–9376. doi: 10.1073/pnas.161191698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shrestha R., Dixon R. A., Chapman K. D. J. Biol. Chem. 2003;278:34990–34997. doi: 10.1074/jbc.M305613200. [DOI] [PubMed] [Google Scholar]
- 15.Shrestha R., Kim S.-C., Dyer J. M., Dixon R. A., Chapman K. D. Biochim. Biophys. Acta. 2006;1761:324–334. doi: 10.1016/j.bbalip.2006.03.004. [DOI] [PubMed] [Google Scholar]
- 16.Schmid M., Davison T. S., Henz S. R., Pape U. J., Demar M., Vingron M., Schölkopf B., Weigel D., Lohmann J. Nat. Genet. 2005;37:501–506. doi: 10.1038/ng1543. [DOI] [PubMed] [Google Scholar]
- 17.Beisson F., Koo A. J. K., Ruuska S., Schwender J., Pollard M., Thelen J., Paddock T., Salas J., Savage L., Milcamps A., et al. Plant Physiol. 2003;132:681–697. doi: 10.1104/pp.103.022988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cravatt B. F., Saghatelian A., Hawkins E. G., Clement A. B., Bracey M. H., Lichtman A. H. Proc. Natl. Acad. Sci. USA. 2004;101:10821–10826. doi: 10.1073/pnas.0401292101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.van der Stelt M., Noordermeer M. A., Kiss T., van Zadelhoff G., Merghart B., Veldink G. A., Vliegenthart J. F. G. Eur. J. Biochem. 2000;267:2000–2007. doi: 10.1046/j.1432-1327.2000.01203.x. [DOI] [PubMed] [Google Scholar]
- 20.Bisogno T., De Petrocellis L., Di Marzo V. Curr. Pharm. Des. 2002;8:533–547. doi: 10.2174/1381612023395655. [DOI] [PubMed] [Google Scholar]
- 21.Tsuboi K., Sun Y. X., Okamoto Y., Araki N., Tonai T., Ueda N. J. Biol. Chem. 2005;280:11082–11092. doi: 10.1074/jbc.M413473200. [DOI] [PubMed] [Google Scholar]
- 22.Alonso J. M., Stepanova A. N., Leisse T. J., Kim C. J., Chen H., Shinn P., Stevenson D. K., Zimmerman J., Barajas P., Cheuk R., et al. Science. 2003;301:653–657. doi: 10.1126/science.1086391. [DOI] [PubMed] [Google Scholar]
- 23.Wang Y.-S., Motes C. M., Mohamalawari D. R., Blancaflor E. B. Cell. Motil. Cytoskeleton. 2004;59:79–93. doi: 10.1002/cm.20024. [DOI] [PubMed] [Google Scholar]
- 24.Curtis M. D., Grossniklaus U. Plant Physiol. 2003;133:462–469. doi: 10.1104/pp.103.027979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dunn K., Dickstein R., Feinbaum R., Burnett B. K., Peterman T. K., Thoidis G., Goodman H. M., Ausubel F. M. Mol. Plant.–Microbe Interact. 1988;2:66–74. doi: 10.1094/mpmi-1-066. [DOI] [PubMed] [Google Scholar]
- 26.An Y.-Q., McDowell J. M., Huang S., McKinney E. C., Chambliss S., Meagher R. B. Plant J. 1996;10:107–121. doi: 10.1046/j.1365-313x.1996.10010107.x. [DOI] [PubMed] [Google Scholar]
- 27.Livak K. J., Schmittgen T. D. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 28.Bradford M. M. Anal. Biochem. 1976;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.