Abstract
SHPS-1 is a transmembrane protein whose extracellular region interacts with CD47 and whose cytoplasmic region undergoes tyrosine phosphorylation and there by binds the protein tyrosine phosphatase SHP-2. Formation of this complex is implicated in regulation of cell migration by an unknown mechanism. A CD47-Fc fusion protein or antibodies to SHPS-1 inhibited migration of human melanoma cells or of CHO cells overexpressing SHPS-1. Overexpression of wild-type SHPS-1 promoted CHO cell migration, whereas expression of the SHPS-1-4F mutant, which lacks the phosphorylation sites required for SHP-2 binding, had no effect. Antibodies to SHPS-1 failed to inhibit migration of CHO cells expressing SHPS-1-4F. SHPS-1 ligands induced the dephosphorylation of SHPS-1 and dissociation of SHP-2. Antibodies to SHPS-1 also enhanced Rho activity and induced both formation of stress fibers and adoption of a less polarized morphology in melanoma cells. Our results suggest that engagement of SHPS-1 by CD47 prevents the positive regulation of cell migration by this protein. The CD47– SHPS-1 system and SHP-2 might thus contribute to the inhibition of cell migration by cell–cell contact.
Keywords: CD47/cell migration/cytoskeletal reorganization/SHP-2/SHPS-1
Introduction
Cell migration is important in a variety of biological processes, including embryonic development, wound healing, inflammation and metastasis. Although the molecular basis of cell migration has not been fully clarified, protein tyrosine phosphorylation and dephosphorylation contribute to its regulation (Anand-Apte and Zetter, 1997). Hepatocyte growth factor and epidermal growth factor, for example, whose receptors possess tyrosine kinase activity, induce the migration of many types of cultured cells (Stoker and Gherardi, 1991). Moreover, the interaction of integrins with extracellular matrix (ECM) proteins, which is essential for cell migration (Hynes and Lander, 1992), results in the tyrosine phosphorylation of various proteins, including those associated directly or indirectly with integrins at focal adhesions (Schaller, 2001).
SHP-2 is a non-transmembrane protein tyrosine phosphatase (PTP) that contains two Src homology 2 (SH2) domains (Matozaki and Kasuga, 1996; Neel and Tonks, 1997). SHP-2 is implicated in the activation of Ras and mitogen-activated protein kinase in response to various growth factors (Noguchi et al., 1994; Bennett et al., 1996), and is therefore thought to positively regulate cell proliferation. In addition, studies with dominant-negative, dominant-active or loss-of-function mutants have demonstrated an important role for SHP-2 in the control of cell migration and cytoskeletal reorganization (Yu et al., 1998; Mañes et al., 1999; Kodama et al., 2000, 2001). The regulation of cell migration by SHP-2 is mediated, at least in part, by the small GTP-binding protein Rho (Kodama et al., 2000, 2001; Schoenwaelder et al., 2000; Lacalle et al., 2002). Thus, SHP-2 is involved in the positive regulation of cell migration.
SHPS-1 (SHP substrate-1) (Fujioka et al., 1996), also known as SIRPα (Kharitonenkov et al., 1997), BIT (Ohnishi et al., 1996), MFR (Saginario et al., 1998) and p84 neural adhesion molecule (Comu et al., 1997), is a member of the immunoglobulin (Ig) superfamily of proteins. The putative extracellular region of SHPS-1 comprises three Ig-like domains with multiple N-linked glycosylation sites, whereas the cytoplasmic region of the protein contains four YXX(L/V/I) motifs, which are putative tyrosine phosphorylation sites and binding sites for the SH2 domains of SHP-2 and SHP-1 (Fujioka et al., 1996; Kharitonenkov et al., 1997; Cant and Ullrich, 2001; Oshima et al., 2002). SHPS-1 is particularly abundant in neurons and macrophages (Comu et al., 1997; Veillette et al., 1998; Seiffert et al., 1999), although other cell types, such as fibroblasts, also express this protein (Fujioka et al., 1996). Tyrosine phosphorylation of SHPS-1 is regulated by various growth factors including insulin and epidermal growth factor (Oshima et al., 2002). Furthermore, adhesion of cultured fibroblasts to various ECM proteins also induces tyrosine phosphorylation of SHPS-1 and its association with SHP-2 in a manner dependent on Src family kinases and focal adhesion kinase (FAK) (Tsuda et al., 1998).
The binding of SHP-2 to the tyrosine-phosphorylated cytoplasmic domain of SHPS-1 increases the PTP activity of SHP-2 in vitro (Ohnishi et al., 1996; Takada et al., 1998). SHPS-1 thus functions to recruit and activate SHP-2 at the cell membrane in response to growth factors or integrin-mediated cell adhesion. Characterization of immortalized fibroblasts from mice that lack most of the cytoplasmic region of SHPS-1 revealed a marked impairment of cell migration associated with an increased formation of actin stress fibers and focal adhesions (Inagaki et al., 2000). These observations suggest that the tyrosine phosphorylation of SHPS-1 and the consequent association of SHPS-1 with SHP-2 promote cell migration through regulation of cytoskeletal reorganization. An SHPS-1-like protein that lacks a cytoplasmic region has also been identified and designated SIRPβ (Kharitonenkov et al., 1997).
CD47, also named IAP, has been implicated as a ligand for SHPS-1 (Jiang et al., 1999; Seiffert et al., 1999). This protein, which was originally identified in association with αvβ3 integrin (Brown et al., 1990), is also a member of the Ig superfamily, possessing an Ig-V-like extracellular domain, five putative membrane-spanning segments and a short cytoplasmic tail (Brown and Frazier, 2001). CD47 and SHPS-1 appear to constitute a cell–cell communication system (the CD47–SHPS-1 system) that plays an important role in a variety of cell functions such as phagocytosis of red blood cells by splenic macrophages (Oldenborg et al., 2000), macrophage multinucleation (Han et al., 2000) and T-cell activation (Seiffert et al., 2001). Neutrophil migration to sites of inflammation is markedly impaired in CD47 knockout mice (Lindberg et al., 1996) and monoclonal antibodies (mAbs) to CD47 inhibit neutrophil transmigration (Cooper et al., 1995; Parkos et al., 1996; Liu et al., 2001), suggesting that CD47 contributes to the regulation of cell migration. However, it has remained unclear whether the binding of CD47 to SHPS-1 regulates cell migration. Furthermore, if this is the case, the downstream signaling components activated by the binding of CD47 to SHPS-1 are unknown. To elucidate the physiological role of the CD47–SHPS-1 system in the regulation of cell migration, we have now examined effects of engagement of SHPS-1 by specific mAbs or a CD47-Fc fusion protein on cell migration.
Results
SHPS-1 ligand-induced inhibition of cell migration
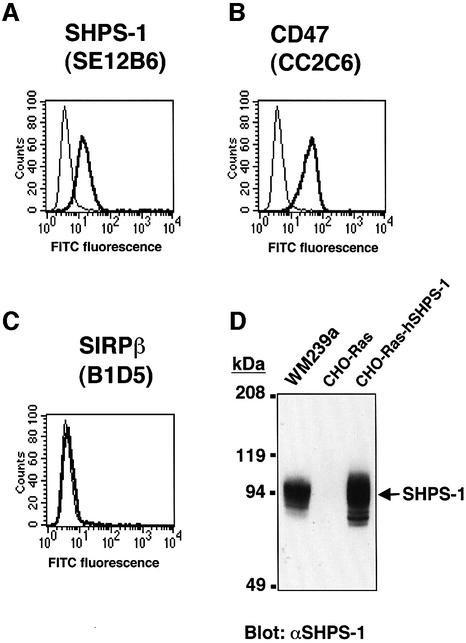
To investigate the effect of CD47 binding to SHPS-1 on cell migration, we examined whether SHPS-1 ligands, including mAbs to SHPS-1 and a recombinant CD47-Fc fusion protein (which contains the extracellular domain of human CD47 fused to the Fc portion of human Ig), modulate the migration of WM239a human melanoma cells across polycarbonate filters. Expression of SHPS-1 on the surface of WM239a cells was demonstrated by flow cytometry (Figure 1A) with the mAbs SE12B6 and SE12C3 (data not shown), which react with the N-terminal Ig-like domain and with the second and third Ig-like domains, respectively, in the extracellular portion of human SHPS-1 (Seiffert et al., 2001). Flow cytometry with a mAb (CC2C6) to human CD47 also revealed expression of CD47 on the surface of WM239a cells (Figure 1B). Surface expression of SIRPβ was not detected (Figure 1C), however, by similar analysis of these cells with the specific mAb B1D5 (Seiffert et al., 2001). Immunoblot analysis of WM239a cell lysates with polyclonal antibodies (pAbs) to SHPS-1 also detected a prominent ∼95 kDa immunoreactive protein (Figure 1D).
Fig. 1. Surface expression of SHPS-1 and CD47 on WM239a melanoma cells. Cells were incubated with the mAbs SE12B6 to SHPS-1 (A), the mAb CC2C6 to CD47 (B), or the mAb B1D5 to SIRPβ (C). Immunocomplexes were then detected with FITC-conjugated goat pAbs to mouse IgG (thick trace) and flow cytometry. The specific mAbs were replaced by normal mouse IgG as a negative control (thin trace). (D) Whole-cell lysates (20 µg of protein) of WM239a cells, of CHO-Ras cells (a negative control), or of CHO-Ras cells stably expressing human SHPS-1 (CHO-Ras-hSHPS-1 cells; a positive control), were subjected to immunoblot analysis with pAbs to SHPS-1 (αSHPS-1). The positions of SHPS-1 and of molecular size standards are indicated. All data are representative of three independent experiments.
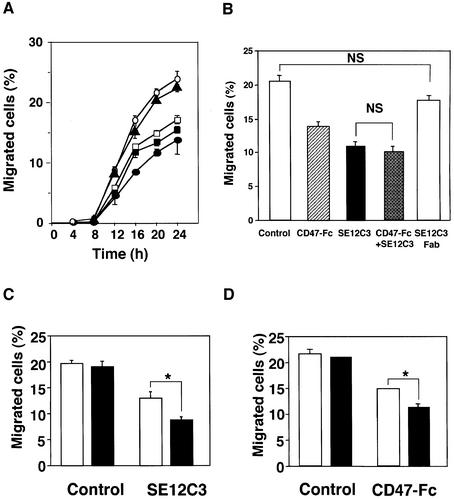
WM239a cells were applied to polycarbonate filters in the upper compartments of a Transwell apparatus, and the number of cells that migrated into the lower compartment was determined at various times thereafter. As shown in Figure 2A, 8 ± 0.4% (mean ± SE, n = 6) of the total applied WM239a cells migrated across the membrane at 12 h and 25 ± 2% at 24 h in the presence of control mouse IgG. However, pretreatment of cells with the SE12C3 inhibited the migration of WM239a cells to 51 ± 2% (n = 3) of control at 12 h and to 56 ± 3% of control (n = 3) at 24 h (Figure 2A). Pretreatment of cells with SE12B6 mAbs also inhibited the migration of WM239a cells to 70 ± 4% (n = 3) of control at 24 h (Figure 2A). The migration of WM239a cells was also inhibited to a similar extent by human CD47-Fc (Figure 2A). The B1D5 mAb specific for SIRPβ had no effect on cell migration (Figure 2A). The inhibitory effects of SE12C3 and human CD47-Fc on WM239a cell migration were concentration dependent, being maximal at 5 µg/ml in both instances (see Supplementary figure 1, available at The EMBO Journal Online). The inhibitory effect of SE12C3 (5 µg/ml) was not further enhanced by the simultaneous presence of human CD47-Fc (10 µg/ml) (Figure 2B), suggesting that both agents might inhibit WM239a cell migration by the same mechanism.
Fig. 2. Effects of SHPS-1 ligands on WM239a cell migration. (A) Cells were preincubated with mAbs SE12C3 (closed circles) or SE12B6 (open squares) to SHPS-1 (5 µg/ml), human CD47-Fc (closed squares; 10 µg/ml), mAb B1D5 (closed triangles) to SIRPβ (5 µg/ml) or control mouse IgG (open circles; 10 µg/ml) for 30 min at 37°C and were then applied to polycarbonate filters in the upper compartments of a Transwell apparatus. After incubation for the indicated times, the number of cells that had migrated into the lower compartments was determined and expressed as a percentage of the total cells applied. (B) Cells were similarly preincubated with control mouse IgG (10 µg/ml), mAb SE12C3 (5 µg/ml), human CD47-Fc (10 µg/ml), SE12C3 (5 µg/ml) plus CD47-Fc (10 µg/ml) or Fab fragments of mAb SE12C3 (5 µg/ml) and then subjected to assay of cell migration for 20 h. (C) Migration assays were performed for 20 h with WM239a cells with control mouse IgG (5 µg/ml) or with mAb SE12C3 (5 µg/ml), each either in the absence (open columns) or presence (solid columns) of goat pAbs to mouse IgG (10 µg/ml). (D) Migration assays were performed for 20 h with cells with control human IgG (10 µg/ml) or with human CD47-Fc (10 µg/ml), each either in the absence (open columns) or presence (solid columns) of goat pAbs to human IgG (10 µg/ml). Data in (A) are means ± SE of triplicates from an experiment that was repeated a total of three times with similar results. Data in (B), (C) and (D) are means ± SE of values from three independent experiments. NS, not significant, *P < 0.05 for the indicated comparisons (Student’s t-test).
Interaction of SHPS-1 ligands with SHPS-1 and effects of cross-linking of SHPS-1 ligands on cell migration
CD47-Fc was shown to exist as a dimer under non-reducing conditions (data not shown), consistent with previous observations (Liu et al., 1993; Suda and Nagata, 1994). Dimer formation is presumably attributable to the formation of a disulfide bond between the Fc portions of the fusion protein. Thus, the interaction of CD47-Fc, like that of mAbs to SHPS-1, with surface SHPS-1 might be expected to result in oligomerization of the latter, and this oligomerization might be responsible for the inhibition of cell migration. To investigate this possibility, we generated WM239a cells that expressed human SHPS-1 fused to green fluorescent protein (hSHPS-1–GFP). Fluorescence microscopy revealed that, in the presence of control mouse IgG, hSHPS-1–GFP was diffusely distributed on the surface of the melanoma cells. In contrast, exposure of cells to mAb SE12C3 or to human CD47-Fc resulted in the clustering of hSHPS-1–GFP molecules on the cell surface (see Supplementary figure 2), suggesting that interaction with its specific ligands induces the surface oligomerization of SHPS-1.
We next examined whether cross-linking of SHPS-1 ligands enhances their inhibitory effect on cell migration. The inhibition of cell migration by mAb SE12C3 (Figure 2C) or by human CD47-Fc (Figure 2D) was indeed potentiated by pAbs either to mouse IgG or to human IgG, respectively. In contrast, Fab fragments of the mAb SE12C3 had no effect on cell migration (Figure 2B). It is thus suggested that the oligomerization of SHPS-1 is involved in the SHPS-1 ligand-induced inhibition of cell migration.
Requirement for SHP-2 binding sites in SHPS-1 ligand-induced inhibition of cell migration
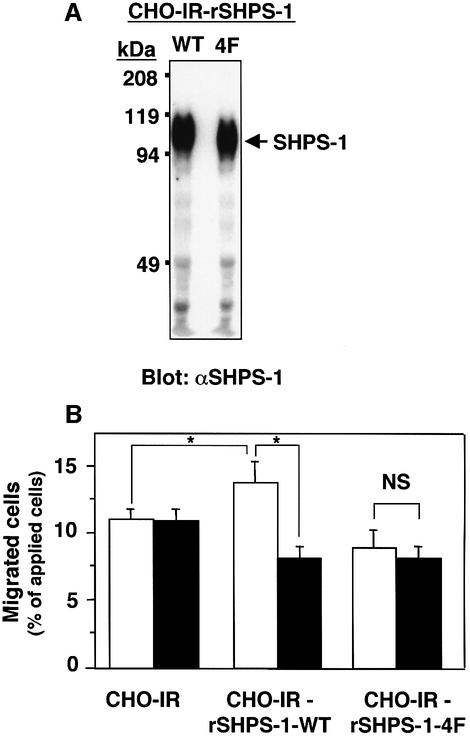
Given that SHPS-1 binds SHP-2 (Fujioka et al., 1996; Kharitonenkov et al., 1997), we next examined whether SHP-2 contributes to the SHPS-1 ligand-induced inhibition of cell migration. For these experiments, we used three CHO cell lines that express human insulin receptors in the absence (CHO-IR cells) or presence of either wild-type rat SHPS-1 (CHO-IR-rSHPS-1-WT cells) or a rat SHPS-1 mutant in which all four tyrosine residues (Tyr408, Tyr432, Tyr449 and Tyr473) in the cytoplasmic domain were replaced by phenylalanine (CHO-IR-rSHPS-1-4F cells) (Takada et al., 1998). It was shown previously that this mutant protein was not tyrosine phosphorylated and did not bind SHP-2 in response to insulin (Takada et al., 1998). Immunoblot analysis of cell lysates with pAbs to SHPS-1 revealed that the amount of rSHPS-1-4F was similar to that of rSHPS-1-WT in their respective cells (Figure 3A). The migration of the three CHO-IR cell lines was minimal in the absence of growth factor (data not shown), but they all showed a marked migratory response to 100 nM insulin. The migratory response of CHO-IR-rSHPS-1-WT cells to insulin was significantly greater than that of the parental CHO-IR cells (Figure 3B). In contrast, the migratory response of CHO-IR-rSHPS-1-4F cells to insulin was similar to that of CHO-IR cells. The mAb 2F34 to rat SHPS-1 markedly inhibited the migration of CHO-IR-rSHPS-1-WT cells but had no effect on that of CHO-IR or CHO-IR-rSHPS-1-4F cells (Figure 3B). These data suggest that complex formation between SHPS-1 and SHP-2 positively regulates cell migration. Furthermore, the formation of such a complex may be required for the inhibition of cell migration by mAb 2F34.
Fig. 3. Requirement for SHP-2 binding sites in the SHPS-1 ligand-induced inhibition of cell migration. (A) Lysates prepared from CHO-IR cells expressing rat wild-type SHPS-1 or from CHO-IR cells expressing the rat SHPS-1-4F mutant were subjected to immunoblot analysis with pAbs to SHPS-1. The position of the recombinant SHPS-1 proteins is indicated. (B) Migration assays were performed for 16 h with parental CHO-IR cells, CHO-IR-rSHPS-1-WT cells, or CHO-IR-rSHPS-1-4F cells that had been preincubated for 30 min at 37°C with normal mouse IgG (5 µg/ml) (open columns) or with mAb 2F34 to rat SHPS-1 (5 µg/ml; solid columns). Insulin (100 nM) was included in the lower chambers of the Transwell apparatus. Data are means ± SE of triplicates from an experiment that was repeated a total of three times with similar results. *P < 0.05 for the indicated comparisons; NS, not significant (Student’s t-test).
Inhibition by SHPS-1 ligands of cell migration in a wound-healing assay
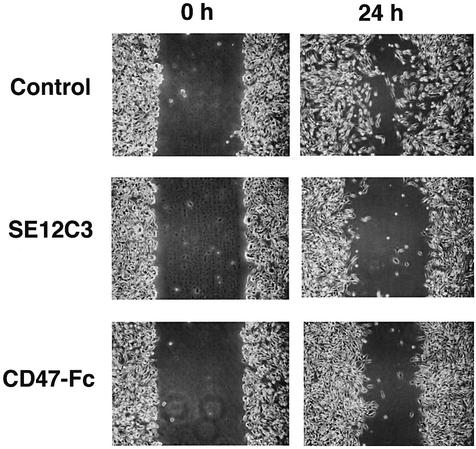
We also examined the effects of SHPS-1 ligands on cell migration in an in vitro assay of wound healing, in which cells migrate unidirectionally from the edge of a scratch wound. A substantial number of WM239a cells had migrated 24 h after the initiation of this assay (Figure 4). Migratory cells exhibited an elongated morphology, with the longitudinal axis oriented toward the wound, a characteristic of well-ordered migration. In contrast, the presence of the SE12C3 mAb to human SHPS-1 or of human CD47-Fc markedly reduced the number of migratory cells apparent 24 h after initiation of the assay (Figure 4).
Fig. 4. Effects of SHPS-1 ligands on cell migration in an assay of wound healing. Monolayers of WM239a cells were wounded and then cultured for 0 or 24 h in the presence of control mouse IgG (5 µg/ml), mAb SE12C3 to human SHPS-1 (5 µg/ml), or human CD47-Fc (10 µg/ml), as indicated. Cell migration into the wound was examined by phase-contrast microscopy (original magnification, ×10). Data are representative of a total of three independent experiments.
Effects of SHPS-1 ligands on tyrosine phosphorylation of SHPS-1
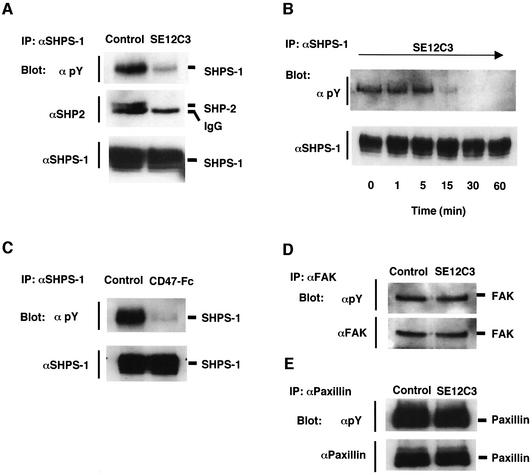
We next examined the effects of mAb SE12C3 and human CD47-Fc on tyrosine phosphorylation of SHPS-1. Tyrosine phosphorylation of SHPS-1 was detected in WM239a cells cultured on a plastic dish in W489 culture medium containing 2% fetal bovine serum (FBS) and insulin (5 µg/ml) (Figure 5A). The combination of cell adhesion, lysophosphatidic acid present in FBS, and insulin may contribute to this level of tyrosine phosphorylation (Fujioka et al., 1996; Tsuda et al., 1998). Incubation of cells with mAb SE12C3 for 30 min resulted in a marked decrease in the extent of tyrosine phosphorylation of SHPS-1 (Figure 5A). The binding of SHP-2 to SHPS-1 was also reduced by treatment of cells with the SE12C3 mAb (Figure 5A). The inhibitory effect of mAb SE12C3 on SHPS-1 phosphorylation was evident within 15 min, maximal at 30 min, and maintained at 60 min (Figure 5B). Similarly, incubation of cells with human CD47-Fc for 30 min also greatly reduced the extent of tyrosine phosphorylation of SHPS-1 (Figure 5C). Given that formation of a complex between SHPS-1 and SHP-2 promotes cell migration (Inagaki et al., 2000) (Figure 3B), these results suggest that SHPS-1 ligands inhibit cell migration by inducing the dephosphorylation of SHPS-1 and the subsequent dissociation of SHP-2 from SHPS-1. We also examined effects of the SE12C3 mAb on tyrosine phosphorylation of FAK and paxillin, given the importance of focal adhesion-associated proteins in cell spreading and migration (Schaller, 2001). However, treatment of cells with mAb SE12C3 did not affect the level of tyrosine phosphorylation of FAK (Figure 5D) or paxillin (Figure 5E).
Fig. 5. Effects of SHPS-1 ligands on tyrosine phosphorylation of SHPS-1 (A–C), on the association of SHPS-1 with SHP-2 (A), and on tyrosine phosphorylation of FAK (D) and paxillin (E). WM239a cells were incubated at 37°C for 30 min (A, D, E) or for indicated times (B) with control mouse IgG (5 µg/ml) or with the SE12C3 mAb to human SHPS-1 (5 µg/ml), as indicated. Alternatively, the cells were incubated with control human IgG (10 µg/ml) or human CD47-Fc (10 µg/ml) for 30 min at 37°C (C). Whole-cell lysates were then prepared and subjected to immunoprecipitation (IP) with the SE12B6 mAb to SHPS-1 (A, B, C), a mAb to FAK (D), or a mAb to paxillin (E). The resulting precipitates were subsequently subjected to immunoblot analysis with either horseradish peroxidase-conjugated mAb PY20 to phosphotyrosine (αpY), a mAb to SHP-2, pAbs to SHPS-1, pAbs to FAK, or a mAb to paxillin, as indicated. Results are representative of three independent experiments.
Effects of SHPS-1 ligation on cell shape, cytoskeletal organization and Rho activity
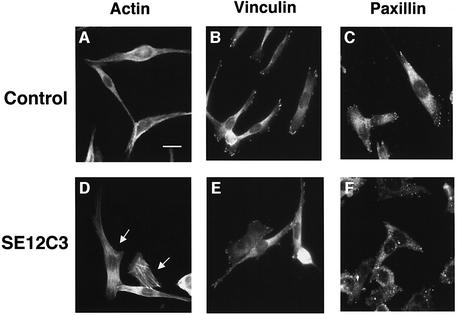
Reorganization of the actin cytoskeleton is required for cell migration (Lauffenburger and Horwitz, 1996; Mitchison and Cramer, 1996). Therefore, we next examined effects of the SE12C3 mAb on cell shape, formation of actin stress fibers and focal adhesions in WM239a cells. Immunofluorescence analysis with rhodamine-labeled phalloidin to detect filamentous actin revealed the melanoma cells to be elongated, highly polarized and spindle-shaped, and to contain few actin stress fibers in the presence of control IgG (Figure 6A). In contrast, ∼50% of cells adopted a polygonal, less polarized morphology and exhibited a marked increase in the number of stress fibers within 30 min of incubation with the mAb SE12C3 (Figure 6D). Immunostaining for vinculin (Figure 6B) and for paxillin (Figure 6C), both of which localize to focal adhesions (Burridge and Mangeat, 1984; Schaller, 2001), revealed immunoreactivity to be prominent at the edges of cells incubated with control IgG. In contrast, vinculin (Figure 6E) and paxillin (Figure 6F) immunoreactivity in cells treated with the SE12C3 mAb was similar to that observed in control cells. These observations thus showed that the SHPS-1 ligand induced both marked changes in cell shape and stress fiber formation in melanoma cells.
Fig. 6. Effects of SHPS-1 ligation on cytoskeletal architecture in WM239a cells. Cells cultured on chamber slides were incubated for 30 min at 37°C with control mouse IgG (5 µg/ml) (A–C) or the SE12C3 mAb to human SHPS-1 (5 µg/ml) (D–F). They were then washed, fixed and stained with rhodamine-conjugated phalloidin (A, D) or with mAbs either to vinculin (B, E) or to paxillin (C, F). Immunocomplexes were detected with FITC- conjugated secondary antibodies. Cells were then examined by fluorescence microscopy. Arrows indicate cells that exhibit a marked change in morphology in response to the SE12C3 mAb. Scale bar: 20 µm. Data are representative of three independent experiments.
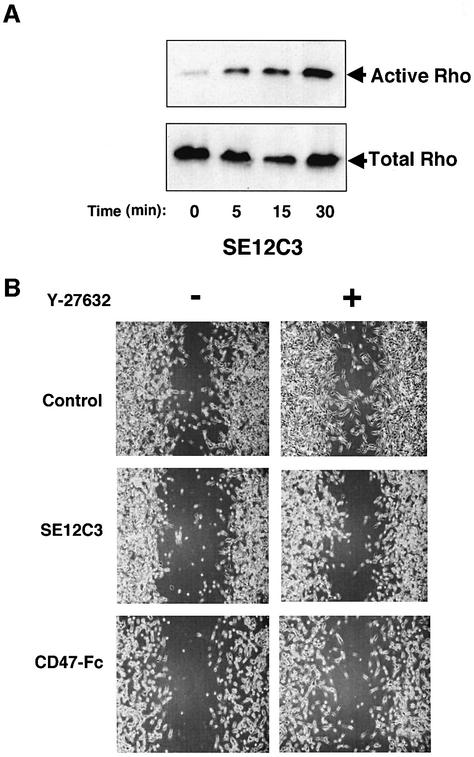
Members of the Rho family of small GTP-binding proteins regulate cell migration through rearrangement of the actin-based cytoskeleton (Hall, 1998; Takai et al., 2001). Moreover, both SHPS-1 and SHP-2 contribute to the regulation of Rho activity (Inagaki et al., 2000; Kodama et al., 2000; Schoenwaelder et al., 2000; Lacalle et al., 2002). We therefore examined whether treatment with the SE12C3 mAb affected the activity of Rho in WM239a cells. The activity of Rho was monitored directly by precipitation of the GTP-bound (activated) form of the protein with a glutathione S-transferase (GST) fusion protein containing the Rho-binding domain of Rhotekin (Inagaki et al., 2000). Incubation of melanoma cells with the SE12C3 mAb resulted in a time-dependent increase in the amount of activated Rho (Figure 7A). These results suggest that activation of Rho may be responsible, at least in part, for morphological changes and impaired migration apparent in WM239a cells exposed to the SE12C3 mAb to human SHPS-1. To test this possibility further, we examined the effect of Y-27632, a p160ROCK inhibitor (Uehata et al., 1997), on inhibition by mAbs to SHPS-1 of cell migration using a wound-healing assay. p160ROCK is a serine/threonine kinase (Ishizaki et al., 1996), which mediates downstream signals of Rho. Treatment of WM239a cells with 10 µM of Y-27632 did not affect cell migration significantly (Figure 7B). In contrast, Y-27632 markedly reversed the inhibition by the mAb SE12C3 or human CD47-Fc of cell migration (Figure 7B). These data suggest that the enhanced activity of Rho is, at least in part, involved in the inhibition by SHPS-1 ligands of cell migration.
Fig. 7. Effects of SHPS-1 ligation on Rho GTPase activity (A) and reversal by Y-27632 of the SHPS-1 ligand-induced inhibition of cell migration (B). (A) Monolayers of WM239a cells at ∼90% confluence were incubated for the indicated times at 37°C with the SE12C3 mAb to human SHPS-l, after which the active form of Rho was precipitated from cell lysates with a GST fusion protein containing the Rho-binding domain of Rhotekin. The resulting precipitates were then subjected to immunoblot analysis with a mAb to RhoA (top panel). Whole-cell lysates were also directly subjected to immunoblot analysis with the same mAb to determine the total amount of Rho (bottom panel). (B) Monolayers of WM239a cells were preincubated with (right panels) or without (left panels) 10 µM Y-27632 for 1 h, wounded and then cultured for 24 h in the presence of control mouse IgG (5 µg/ml; top panels), the SE12C3 mAb to human SHPS-1 (5 µg/ml; middle panels), or the human CD47-Fc (10 µg/ml; bottom panels), as indicated. Cell migration into the wound was examined by phase-contrast microscopy (original magnification, ×10). Results are representative of three independent experiments.
Discussion
We have shown that mAbs to human SHPS-1 or a human CD47-Fc fusion protein inhibit the migration of WM239a melanoma cells, which express SHPS-1, in either a Transwell assay or a wound-healing assay. The maximal inhibitory effect of CD47-Fc was not further enhanced by the presence of mAbs to SHPS-1, suggesting that these SHPS-1 ligands inhibit cell migration by the same mechanism. Furthermore, the inhibitory effect of either mAbs to SHPS-1 or CD47-Fc on cell migration was enhanced by cross-linking of these SHPS-1 ligands with secondary Abs. In contrast, Fab fragments of the mAb to SHPS-1 had no effect on cell migration. Monoclonal Abs to human SHPS-1 or to rat SHPS-1 also inhibited the migration of CHO-Ras cells stably expressing recombinant human or rat SHPS-1, respectively, without affecting that of parental CHO-Ras cells; these mAbs did not react with endogenous hamster SHPS-1 in CHO-Ras cells (see Supplementary figure 3). Although WM239a melanoma cells express both SHPS-1 and CD47, our results indicate that the inhibition of cell migration by SHPS-1 ligands is due to the engagement of surface SHPS-1, not to disruption of the interaction between SHPS-1 and CD47 on either a single melanoma cell or adjacent melanoma cells.
During the course of this study, Liu et al. (2002) demonstrated that mAbs to SHPS-1 or soluble fusion proteins containing the extracellular domain of CD47 inhibited the migration of neutrophils across an epithelial cell layer. These researchers also showed that the SHPS-1 ligands inhibited the migration of individual cells, although they did not provide evidence as to the mechanism by which ligand binding to SHPS-1 inhibited neutrophil migration. We have now shown that expression of wild-type SHPS-1, but not that of an SHPS-1 mutant (SHPS-1-4F) that does not bind SHP-2, promotes the migration of CHO-IR cells in response to insulin. Consistent with these observations, the migration of immortalized fibroblasts from mice that lack most of the cytoplasmic region of SHPS-1 was shown previously to be markedly impaired (Inagaki et al., 2000). In addition, SHP-2 has been shown to contribute to growth factor-stimulated or integrin-mediated cell migration (Yu et al., 1998; Manes et al., 1999; Kodama et al., 2000). Our present results indicate that complex formation between SHPS-1 and SHP-2 promotes cell migration. Our observation that mAbs to rat SHPS-1 inhibited the migration of CHO-IR cells expressing wild-type rat SHPS-1 but not that of CHO-IR cells expressing the rat SHPS-1-4F mutant further indicates that formation of the SHPS-1–SHP-2 complex is required for inhibition of cell migration by mAbs to SHPS-1.
We have demonstrated that exposure of cells to mAbs to SHPS-1 or to CD47-Fc induces dephosphorylation of SHPS-1 and dissociation of SHP-2 from SHPS-1. This suggests that the effect of SHPS-1 ligands on cell migration is due to the impaired complex formation between SHPS-1 and SHP-2, because this complex formation positively regulates cell migration, presumably through recruitment and activation of SHP-2 at the plasma membrane as mentioned above. In addition, expression of a dominant-negative mutant of SHP-2 also resulted in impairment of cell migration, increased stress fiber formation and an increase of Rho activity (Yu et al., 1998; Mañes et al., 1999; Kodama et al., 2000), consistent with the notion that dissociation of SHP-2 from SHPS-1 (namely, functional inactivation of SHP-2) participates in inhibition of cell migration and cytoskeletal disorganization in response to SHPS-1 ligands.
Engagement of integrins by the ECM results in tyrosine phosphorylation of SHPS-1 as well as of focal adhesion-associated proteins such as FAK and paxillin (Tsuda et al., 1998; Oshima et al., 2002). However, the tyrosine phosphorylation of FAK or paxillin was not affected by SHPS-1 ligands, suggesting that dephosphorylation of SHPS-1 induced by SHPS-1 ligands is not a secondary effect of the disruption of focal adhesions. The mechanism of dephosphorylation of SHPS-1 by its ligands is unknown. An inhibitor of PTPs abolished the dephosphorylation of SHPS-1 by its ligand (see Supplementary figure 4), suggesting that a PTP is involved, at least in part, in this process. Therefore, it is possible that engagement of SHPS-1 by its ligands induces oligomerization of SHPS-1 complexed with SHP-2, which might then result in the dephosphorylation of SHPS-1 in trans. Alternatively, engagement of SHPS-1 might induce its translocation to a cellular compartment that prevents its interaction with tyrosine kinases, such as Src family kinases, responsible for its tyrosine phosphorylation (Tsuda et al., 1998).
Treatment of WM239a melanoma cells with mAbs to SHPS-1 induced cells to adopt a less polarized, epithelial cell-like morphology with an increased number of actin stress fibers. This phenotype partly resembles that of fibroblasts derived from mice that lack most of the cytoplasmic region of SHPS-1 (Inagaki et al., 2000). Activated Rho induces increased stress fiber formation (Hall, 1998; Takai et al., 2001). We demonstrate here that the treatment of WM239a melanoma cells with mAbs to SHPS-1 increased the activity of Rho, consistent with the observation that the same mAb increased the formation of actin stress fibers. Furthermore, the impaired cell migration in response to SHPS-1 mAbs or CD47-Fc was reversed by a p160ROCK inhibitor. Together, our present results indicate that engagement of surface SHPS-1 by specific ligands inhibits cell migration by enhancing the activity of Rho and thus impairing cytoskeletal reorganization.
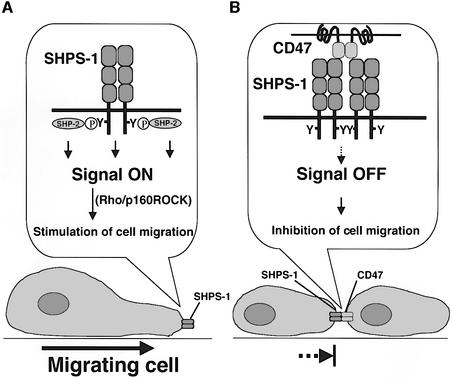
We thus propose a model for the regulation of cell migration by the CD47–SHPS-1 system (Figure 8). During the migration of an SHPS-1-expressing cell, SHPS-1 is tyrosine-phosphorylated, in response either to integrin engagement by the ECM or to growth factor stimulation, and consequently forms a complex with SHP-2. This complex formation is crucial for positive regulation of cell migration, which is presumably mediated by downstream signaling by Rho. CD47 has been suggested to form dimers in cis (Brown et al., 1990; Parkos et al., 1996), and we have shown that SHPS-1 also forms a cis-dimer (R.Sato, H.Ohnishi and T.Matozaki, unpublished data). Thus, when a migrating SHPS-1-expressing cell makes contact with a nearby CD47-expressing cell, the interaction of CD47 with SHPS-1 in trans is likely to result. In fact, CHO-Ras-hSHPS-1 cells and CHO-Ras-hCD47 formed a cell–cell contact, where SHPS-1 and CD47 were co-localized, suggesting the interaction between these two proteins (see Supplementary figure 5). This interaction is mimicked by the binding either of specific mAbs or of CD47-Fc to SHPS-1 in the present study. Engagement of cis-dimers of SHPS-1 then results in dephosphorylation of SHPS-1, the dissociation of SHP-2 from SHPS-1, an enhancement in Rho activity, impairment of cytoskeletal reorganization and inhibition of cell migration.
Fig. 8. Model for the regulation of cell migration by the CD47–SHPS-1 system. (A) During migration of an SHPS-1-expressing cell, SHPS-1 is tyrosine-phosphorylated in response either to integrin engagement by the ECM or to growth factor stimulation, resulting in the formation of an SHPS-1–SHP-2 complex that positively regulates cell migration through downstream signaling components including Rho and p160ROCK. (B) Interaction of a migratory SHPS-1-expressing cell with an adjacent CD47-expressing cell results in the engagement of SHPS-1 dimers by CD47 and consequent dephosphorylation of SHPS-1, the dissociation of SHP-2 from SHPS-1, impairment of Rho regulation and cytoskeletal reorganization, and inhibition of cell migration.
Several different mAbs to CD47 have been shown to inhibit neutrophil migration across cell monolayers (Cooper et al., 1995; Parkos et al., 1996; Liu et al., 2001). The CC2C6 mAb to CD47 also inhibited the migration of WM239a melanoma cells (S.Motegi, H.Okazawa and T.Matozaki, unpublished data). The binding of SHPS-1 to CD47 might thus inhibit migration in cells expressing CD47, suggesting that the CD47–SHPS-1 system might mediate bidirectional inhibitory regulation of cell migration. Such a scenario resembles the bidirectional negative regulation of T cells and dendritic cells by the CD47-SHPS-1 system (Latour et al., 2001).
Cell–cell contact has long been known to contribute to inhibition of cell migration and growth, although the molecular mechanism of these effects has remained unclear (Fagotto and Gumbiner, 1996). Both SHPS-1 and CD47 are expressed in many cell types, although SHPS-1 is especially abundant in neurons and macrophages (Comu et al., 1997; Veillette et al., 1998). The CD47–SHPS-1 system might thus contribute widely to the inhibition of cell migration by cell–cell contact. It is also possible that the CD47–SHPS-1 system is important for the inhibition of cell growth induced by cell–cell contact.
Materials and methods
Primary antibodies
Mouse mAbs to human SHPS-1 (SE12C3, SE12B6), to human CD47 (CC2C6) and to human SIRPβ (B1D5) were generated and purified as described previously (Seiffert et al., 1999, 2001), as was a mouse mAb to rat SHPS-1 (2F34) (Takada et al., 1998). Rabbit pAbs to SHPS-1 were obtained from Millennium; a mouse mAb to vinculin was from Sigma; mouse mAbs to FAK and to paxillin were from Upstate Biotechnology; a horseradish peroxidase-conjugated mouse mAb (PY20) to phospho tyrosine, mouse mAbs to RhoA and to SHP-2, and rabbit pAbs to FAK were from Santa Cruz Biotechnology. To prepare Fab fragments of SE12C3, we treated the mAb with papain using ImmunoPure Fab Preparation Kit (PIERCE) according to the manufacturer’s protocol.
Cells and cell culture
All cells were maintained at 37°C under a humidified atmosphere of 5% CO2 in air. The human melanoma cell line WM239a was kindly provided by M.Herlyn (Wistar Institute, Philadelphia, PA). WM239a cells were maintained in medium W489, a 4:1 (v/v) mixture of MCDB153 (Sigma) and L15 (Gibco-BRL), supplemented with 2 mM CaCl2, 2% FBS (Gibco-BRL) and insulin (5 µg/ml). CHO cells stably expressing H-Ras (CHO-Ras cells) (Katakura et al., 1999) were kindly provided by S.Shirahata (Kyushu University, Fukuoka, Japan). CHO cells stably expressing human insulin receptors (CHO-IR cells) and CHO-Ras cells were cultured in αMEM (Sigma) supplemented with 2 mM l-glutamine, 10 mM HEPES–NaOH pH 7.6 and 10% FBS. CHO-IR cells stably expressing either rat wild-type SHPS-1 (CHO-IR-rSHPS-1-WT cells) or the rat SHPS-1-4F mutant (CHO-IR-rSHPS-1-4F cells) were generated previously (Takada et al., 1998) and cultured as described above for parental cells.
Preparation of CD47-Fc fusion proteins
Human CD47 cDNA was kindly provided by F.P.Lindberg (Washington University, St Louis, MO). To prepare a human CD47-Fc fusion protein, we first obtained a cDNA that encodes the Fc portion of human IgG1 by PCR as described previously (Liu et al., 1993). The PCR product was then digested with SpeI and NotI and ligated into the corresponding sites of the pEFneo vector (Invitrogen), yielding pEFneoFc76. A cDNA encoding the extracellular domain of human CD47 (amino acids 1–142) was amplified from the full-length human CD47 cDNA by PCR with the sense primer 5′-TAATACTAGTCTGCTGCTCCAGACACCTGCG-3′ and the antisense primer 5′-TAGCTCTAGAATTTTCATTTGGAGAAAACCATG AAAC-3′. The PCR product was digested with SpeI and XbaI and ligated into SpeI site of pEFneoFc76. The resulting construct was digested with PstI and NotI, and the released fragment was then subcloned into the corresponding sites of pTracerCMV. CHO-Ras cells were transfected with the resulting pTracerCMV-hCD47-Fc plasmid and subjected to selection with Zeocin as described above. Several cell lines producing human CD47-Fc were identified by immunoblot analysis of culture supernatants with pAbs to human IgG. The CD47-Fc fusion protein was then purified from such culture supernatants by column chromatography on rProtein A–Sepharose 4FF (Amersham Pharmacia Biotech).
Flow cytometry
The surface expression of SHPS-1, CD47 and SIRPβ on WM239a cells was examined by flow cytometry as described previously (Kaneko et al., 1996). In brief, cells were detached from culture dishes by treatment with 0.01% EDTA and then washed in phosphate-buffered saline (PBS). They (0.5 × 106 to 1 × 106) were then incubated with primary Abs (2 µg/ml) for 30 min on ice, washed twice with PBS, and incubated for 30 min on ice with fluorescein isothiocyanate (FITC)-conjugated goat pAbs to mouse IgG (Immunotech). After washing twice with PBS, stained cells were suspended in 1 ml of PBS and analyzed with a FACSCalibur flow cytometer (Becton Dickinson, San Jose, CA). Data were processed with CellQuest software (Becton Dickinson).
Cell migration assay
Cell migration was assayed with a Transwell apparatus (Corning) as described previously (Longo et al., 2001). In brief, cells were detached from culture dishes, resuspended in culture medium [W489 containing 2% FBS and insulin (5 µg/ml) for WM239a cells; αMEM containing 10% FBS for CHO cells], and then preincubated for 30 min at 37°C with mAbs to SHPS-1, with CD47-Fc, or with control IgG. A portion of the cell suspension (1 × 105 cells in 100 µl) was then transferred to a polycarbonate filter (pore size, 8 µm; Corning) in the upper compartment of a Transwell apparatus, and 600 µl of fresh culture medium were placed in the lower compartment. For assay of CHO-IR cell migration, 100 nM insulin was also added to the medium in the lower compartment. The apparatus was then placed for the indicated times at 37°C in a humidified incubator containing 5% CO2. The number of cells that had migrated into the lower compartment was then counted in triplicate with a hemocytometer and was expressed as a percentage of the total number of cells added to the upper compartment.
Wound-healing assay
WM239a cells were grown in 60 mm culture dishes until confluent, and the cell monolayer was then wounded by scoring with a sterile plastic 200 µl micropipette tip. In the case of treatment with Y-27632 (Calbiochem), cells were preincubated with this compound for 1 h before wounding. The cells were washed once with PBS and incubated under a humidified atmosphere of 5% CO2 for 24 h at 37°C in W489 medium containing 2% FBS and insulin (5 µg/ml). They were then examined by phase-contrast microscopy.
Immunoprecipitation and immunoblot analysis
Cells (∼1 × 107) were washed with ice-cold PBS and then lysed on ice in 1 ml of lysis buffer [20 mM Tris–HCl pH 7.6, 140 mM NaCl, 1 mM EDTA, 1% Nonidet P-40] containing 1 mM phenylmethylsulfonyl fluoride, aprotinin (10 µg/ml) and 1 mM sodium vanadate. The lysates were centrifuged at 10 000 g for 15 min at 4°C, and the resulting supernatants were subjected to immunoprecipitation and immunoblot analysis, performed as described previously (Tsuda et al., 1998).
Immunofluorescence analysis
Cells seeded on Lab-tek chamber slides (Nalge Nunc) were incubated with the SE12C3 mAb (5 µg/ml) or normal mouse IgG (5 µg/ml) for 30 min at 37°C, washed with PBS, fixed for 20 min with 4% formaldehyde in PBS, permeabilized for 5 min with 0.5% Triton X-100 in PBS, and incubated for 2 h at room temperature in TBS-T [20 mM Tris–HCl pH 7.6, 150 mM NaCl, 0.05% Tween-20] containing 5% non-fat dried milk, 10% FBS and 1% bovine serum albumin. The cells were then incubated for 1 h at room temperature with a mAb to vinculin (20 µg/ml), a mAb to paxillin (5 µg/ml), or rhodamine–phalloidin (0.1 µg/ml) (Sigma); in the case of staining for vinculin or paxillin, the cells were washed twice with PBS and incubated for 30 min with FITC-conjugated sheep pAbs to mouse Ig (Amersham Pharmacia Biotech). After three washes with PBS, the cells were examined with an AX-70 microscope equipped with epifluorescence optics (Olympus, Tokyo, Japan).
Assay of activated Rho
Activated Rho was assayed as described previously (Inagaki et al., 2000). In brief, cells in one 100 mm dish were lysed in 600 µl of a solution containing 25 mM HEPES–NaOH pH 7.4, 100 mM NaCl, 0.5% Nonidet P-40, 10 mM MgCl2, 10 mM β-glycerophosphate, 10% glycerol, 1 mM phenylmethylsulfonyl fluoride, leupeptin (10 µg/ml) and aprotinin (10 µg/ml). Cell lysates were then incubated for 45 min at 4°C with a GST fusion protein that contained the Rho-binding domain (amino acids 7–89) of mouse Rhotekin and was bound to glutathione–Sepharose beads (Amersham Pharmacia Biotech). Proteins that bound to the beads were subjected to immunoblot analysis with a mAb specific for RhoA. The total abundance of Rho was also determined by immunoblot analysis of cell lysates.
Statistical analysis
Statistical analysis was performed by Student’s t-test with the use of Stat View 5.0 software (SAS Institute). A P value of < 0.05 was considered statistically significant.
Supplementary data
Supplementary data are available at The EMBO Journal Online.
Acknowledgments
Acknowledgements
We thank M.Herlyn for providing WM239a cells, S.Shirahata for CHO-Ras clone 1, F.P.Lindberg for human CD47 cDNA, T.Noguchi for helpful discussion and K.Shimofure for technical assistance and secretarial work. This work was supported by a Grant-in-Aid for Scientific Research on Priority Areas Cancer, a Grant-in-Aid for Scientific Research (B) and a Grant-in-Aid for the 21st Century COE Program from the Ministry of Education, Culture, Sports, Science and Technology of Japan; a grant from the Yamanouchi Foundation for Research on Metabolic Disorders; a grant from the Novartis Foundation (Japan) for the Promotion of Science; a grant from ONO Medical Research Foundation; a grant from the Cosmetology Research Foundation; and a grant from Mitsui Life Social Welfare Foundation.
References
- Anand-Apte B. and Zetter,B. (1997) Signaling mechanisms in growth factor-stimulated cell motility. Stem Cells, 15, 259–267. [DOI] [PubMed] [Google Scholar]
- Bennett A.M., Hausdorff,S.F., O’Reilly,A.M., Freeman,R.M. and Neel,B.G. (1996) Multiple requirements for SHPTP2 in epidermal growth factor-mediated cell cycle progression. Mol. Cell. Biol., 16, 1189–1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown E.J. and Frazier,W.A. (2001) Integrin-associated protein (CD47) and its ligands. Trends Cell Biol., 11, 130–135. [DOI] [PubMed] [Google Scholar]
- Brown E., Hooper,L., Ho,T. and Gresham,H. (1990) Integrin-associated protein: a 50-kD plasma membrane antigen physically and functionally associated with integrins. J. Cell Biol., 111, 2785–2794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burridge K. and Mangeat,P. (1984) An interaction between vinculin and talin. Nature, 308, 744–746. [DOI] [PubMed] [Google Scholar]
- Cant C.A. and Ullrich,A. (2001) Signal regulation by family conspiracy. Cell. Mol. Life Sci., 58, 117–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comu S., Weng,W., Olinsky,S., Ishwad,P., Mi,Z., Hempel,J., Watkins, S., Lagenaur,C.F. and Narayanan,V. (1997) The murine P84 neural adhesion molecule is SHPS-1, a member of the phosphatase-binding protein family. J. Neurosci., 17, 8702–8710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper D., Lindberg,F.P., Gamble,J.R., Brown,E.J. and Vadas,M.A. (1995) Transendothelial migration of neutrophils involves integrin-associated protein (CD47). Proc. Natl Acad. Sci. USA, 92, 3978–3982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagotto F. and Gumbiner,B.M.(1996) Cell contact-dependent signaling. Dev. Biol., 180, 445–454. [DOI] [PubMed] [Google Scholar]
- Fujioka Y., Matozaki,T., Noguchi,T., Iwamatsu,A., Yamao,T., Takahashi,N., Tsuda,M., Takada,T. and Kasuga,M. (1996) A novel membrane glycoprotein, SHPS-1, that binds the SH2-domain-containing protein tyrosine phosphatase SHP-2 in response to mitogens and cell adhesion. Mol. Cell. Biol., 16, 6887–6899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han X., Sterling,H., Chen,Y., Saginario,C., Brown,E.J., Frazier,W.A., Lindberg,F.P. and Vignery,A. (2000) CD47, a ligand for the macrophage fusion receptor, participates in macrophage multinucleation. J. Biol. Chem., 275, 37984–37992. [DOI] [PubMed] [Google Scholar]
- Hall A. (1998) Rho GTPases and the actin cytoskeleton. Science, 279, 509–514. [DOI] [PubMed] [Google Scholar]
- Hynes R.O. and Lander,A.D. (1992) Contact and adhesive specificities in the associations, migrations, and targeting of cells and axons. Cell, 68, 303–322. [DOI] [PubMed] [Google Scholar]
- Inagaki K., Yamao,T., Noguchi,T., Matozaki,T., Fukunaga,K., Takada,T., Hosooka,T., Akira,S. and Kasuga,M. (2000) SHPS-1 regulates integrin-mediated cytoskeletal reorganization and cell motility. EMBO J., 19, 6721–6731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishizaki T. et al. (1996) The small GTP-binding protein Rho binds to and activates a 160 kDa Ser/Thr protein kinase homologous to myotonic dystrophy kinase. EMBO J., 15, 1885–1893. [PMC free article] [PubMed] [Google Scholar]
- Jiang P., Lagenaur,C.F. and Narayanan,V. (1999) Integrin-associated protein is a ligand for the P84 neural adhesion molecule. J. Biol. Chem., 274, 559–562. [DOI] [PubMed] [Google Scholar]
- Kaneko Y., Hirose,S., Abe,M., Yagita,H., Okumura,K. and Shirai,T. (1996) CD40-mediated stimulation of B1 and B2 cells: implication in autoantibody production in murine lupus. Eur. J. Immunol., 12, 3061–3065. [DOI] [PubMed] [Google Scholar]
- Katakura Y., Seto,P., Miura,T., Ohashi,H., Teruya,K. and Shirahata,S. (1999) Productivity enhancement of recombinant protein in CHO cells via specific promoter activation by oncogenes. Cytotechnology, 31, 103–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kharitonenkov A., Chen,Z., Sures,I., Wang,H., Schilling,J. and Ullrich,A. (1997) A family of proteins that inhibit signalling through tyrosine kinase receptors. Nature, 386, 181–186. [DOI] [PubMed] [Google Scholar]
- Kodama A., Matozaki,T., Fukuhara,A., Kikyo,M., Ichihashi,M. and Takai,Y. (2000) Involvement of an SHP-2-Rho small G protein pathway in hepatocyte growth factor/scatter factor-induced cell scattering. Mol. Biol. Cell, 11, 2565–2575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kodama A., Matozaki,T., Shinohara,M., Fukuhara,A., Tachibana,K., Ichihashi,M., Nakanishi,H. and Takai,Y. (2001) Regulation of Ras and Rho small G proteins by SHP-2. Genes Cells, 6, 869–876. [DOI] [PubMed] [Google Scholar]
- Lacalle R.A., Mira,E., Gomez-Mouton,C., Jimenez-Baranda,S., Martinez-A.C., Manes,S. (2002) Specific SHP-2 partitioning in raft domains triggers integrin-mediated signaling via Rho activation. J. Cell Biol., 157, 277–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latour S. et al. (2001) Bidirectional negative regulation of human T and dendritic cells by CD47 and its cognate receptor signal-regulator protein-alpha: down-regulation of IL-12 responsiveness and inhibition of dendritic cell activation. J. Immunol., 167, 2547–2554. [DOI] [PubMed] [Google Scholar]
- Lauffenburger D.A. and Horwitz,A.F. (1996) Cell migration: a physi cally integrated molecular process. Cell, 84, 359–369. [DOI] [PubMed] [Google Scholar]
- Lindberg F.P., Bullard,D.C., Caver,T.E., Gresham,H.D., Beaudet,A.L. and Brown,E.J. (1996) Decreased resistance to bacterial infection and granulocyte defects in IAP-deficient mice. Science, 274, 795–798. [DOI] [PubMed] [Google Scholar]
- Liu Y.C., Kawagishi,M., Mikayama,T., Inagaki,Y., Takeuchi,T. and Ohashi,H. (1993) Processing of a fusion protein by endoprotease in COS-1 cells for secretion of mature peptide by using a chimeric expression vector. Proc. Natl Acad. Sci. USA, 90, 8957–8961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Merlin,D., Burst,S.L., Pochet,M., Madara,J.L. and Parkos,C.A. (2001) The role of CD47 in neutrophil transmigration. Increased rate of migration correlates with increased cell surface expression of CD47. J. Biol. Chem., 276, 40156–40166. [DOI] [PubMed] [Google Scholar]
- Liu Y., Bühring,H.J., Zen,K., Burst,S.L., Schnell,F.J., Williams,I.R. and Parkos,C.A. (2002) Signal regulatory protein (SIRPα), a cellular ligand for CD47, regulates neutrophil transmigration. J. Biol. Chem., 277, 10028–10036. [DOI] [PubMed] [Google Scholar]
- Longo N., Yanez-Mo,M., Mittelbrunn,M., de la Rosa,G., Munoz,M.L., Sanchez-Madrid,F. and Sanchez-Mateos,P. (2001) Regulatory role of tetraspanin CD9 in tumor–endothelial cell interaction during transendothelial invasion of melanoma cells. Blood, 98, 3717–3726. [DOI] [PubMed] [Google Scholar]
- Mañes S., Mira,E., Gómez-Mouton,C., Zhao,Z.J., Lacalle,R.A. and Martínez,A.C. (1999) Concerted activity of tyrosine phosphatase SHP-2 and focal adhesion kinase in regulation of cell motility. Mol. Cell. Biol., 19, 3125–3135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matozaki T. and Kasuga,M. (1996) Roles of protein-tyrosine phosphatases in growth factor signalling. Cell Signal., 8, 13–19. [DOI] [PubMed] [Google Scholar]
- Mitchison T.J. and Cramer,L.P. (1996) Actin-based cell motility and cell locomotion. Cell, 84, 371–379. [DOI] [PubMed] [Google Scholar]
- Neel B.G and Tonks,N.K. (1997) Protein tyrosine phosphatases in signal transduction. Curr. Opin. Cell Biol., 9, 193–204. [DOI] [PubMed] [Google Scholar]
- Noguchi T., Matozaki,T., Horita,K., Fujioka,Y. and Kasuga,M. (1994) Role of SH-PTP2, a protein-tyrosine phosphatase with Src homology 2 domains, in insulin-stimulated Ras activation. Mol. Cell. Biol., 14, 6674–6682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohnishi H., Kubota,M., Ohtake,A., Sato,K. and Sano,S. (1996) Activation of protein-tyrosine phosphatase SH-PTP2 by a tyrosine-based activation motif of a novel brain molecule. J. Biol. Chem., 271, 25569–25574. [DOI] [PubMed] [Google Scholar]
- Oldenborg P.A., Zheleznyak,A., Fang,Y.F., Lagenaur,C.F., Gresham,H.D. and Lindberg,F.P. (2000) Role of CD47 as a marker of self on red blood cells. Science, 288, 2051–2054. [DOI] [PubMed] [Google Scholar]
- Oshima K., Ruhul Amin,A.R., Suzuki,A., Hamaguchi,M. and Matsuda,S. (2002) SHPS-1, a multifunctional transmembrane glycoprotein. FEBS Lett., 519, 1–7. [DOI] [PubMed] [Google Scholar]
- Parkos C.A., Colgan,S.P., Liang,T.W., Nusrat,A., Bacarra,A.E., Carnes,D.K. and Madara,J.L. (1996) CD47 mediates post-adhesive events required for neutrophil migration across polarized intestinal epithelia. J. Cell Biol., 132, 437–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saginario C., Sterling,H., Beckers,C., Kobayashi,R., Solimena,M., Ullu,E. and Vignery,A. (1998) MFR, a putative receptor mediating the fusion of macrophages. Mol. Cell. Biol., 18, 6213–6223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaller M.D. (2001) Biochemical signals and biological responses elicited by the focal adhesion kinase. Biochim. Biophys. Acta, 1540, 1–21. [DOI] [PubMed] [Google Scholar]
- Schoenwaelder S.M., Petch,L.A., Williamson,D., Shen,R., Feng,G.S. and Burridge,K. (2000) The protein tyrosine phosphatase Shp-2 regulates RhoA activity. Curr. Biol., 10, 1523–1526. [DOI] [PubMed] [Google Scholar]
- Seiffert M., Cant,C., Chen,Z., Rappold,I., Brugger,W., Kanz,L., Brown,E.J., Ullrich,A. and Bühring,H.J. (1999) Human signal-regulatory protein is expressed on normal, but not on subsets of leukemic myeloid cells and mediates cellular adhesion involving its counterreceptor CD47. Blood, 94, 3633–3643. [PubMed] [Google Scholar]
- Seiffert M., Brossart,P., Cant,C., Cella,M., Colonna,M., Brugger,W., Kanz,L., Ullrich,A. and Bühring,H.J. (2001) Signal-regulatory protein α (SIRPα) but not SIRPβ is involved in T-cell activation, binds to CD47 with high affinity, and is expressed on immature CD34+CD38– hematopoietic cells. Blood, 97, 2741–2749. [DOI] [PubMed] [Google Scholar]
- Stoker M. and Gherardi,E. (1991) Regulation of cell movement: the motogenic cytokines. Biochim. Biophys. Acta, 1072, 81–102. [DOI] [PubMed] [Google Scholar]
- Suda T. and Nagata,S. (1994) Purification and characterization of the Fas-ligand that induces apoptosis. J. Exp. Med., 179, 873–879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takada T. et al. (1998) Roles of the complex formation of SHPS-1 with SHP-2 in insulin-stimulated mitogen-activated protein kinase activation. J. Biol. Chem., 273, 9234–9242. [DOI] [PubMed] [Google Scholar]
- Takai Y., Sasaki,T. and Matozaki,T. (2001) Small GTP-binding proteins. Physiol. Rev., 81, 153–208. [DOI] [PubMed] [Google Scholar]
- Tsuda M. et al. (1998) Integrin-mediated tyrosine phosphorylation of SHPS-1 and its association with SHP-2. Roles of Fak and Src family kinases. J. Biol. Chem., 273, 13223–13229. [DOI] [PubMed] [Google Scholar]
- Uehata M. et al. (1997) Calcium sensitization of smooth muscle mediated by a Rho-associated protein kinase in hypertension. Nature, 389, 990–994. [DOI] [PubMed] [Google Scholar]
- Veillette A., Thibaudeau,E. and Latour,S. (1998) High expression of inhibitory receptor SHPS-1 and its association with protein-tyrosine phosphatase SHP-1 in macrophages. J. Biol. Chem., 273, 22719–22728. [DOI] [PubMed] [Google Scholar]
- Yu D.H., Qu,C.K., Henegariu,O., Lu,X. and Feng,G.S. (1998) Protein-tyrosine phosphatase Shp-2 regulates cell spreading, migration, and focal adhesion. J. Biol. Chem., 273, 21125–21131. [DOI] [PubMed] [Google Scholar]