Abstract
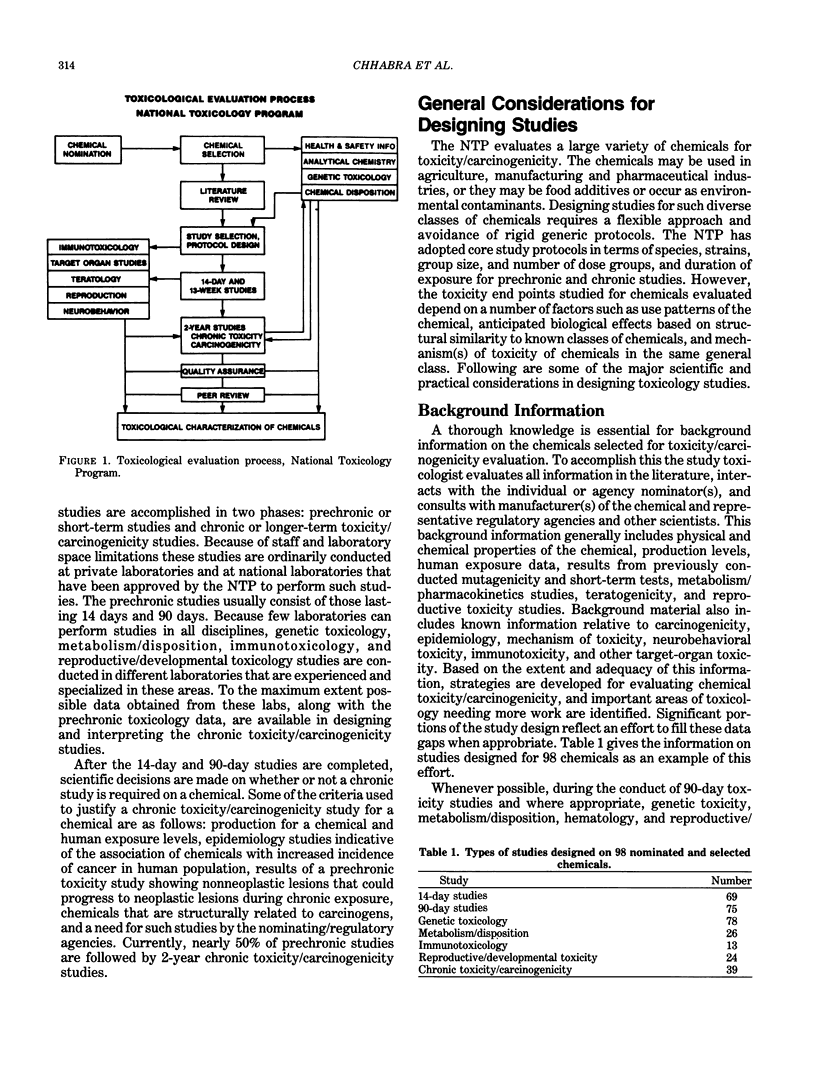
Since the establishment of the National Toxicology Program (NTP), there have been gradual changes in strategies to evaluate the overall toxicity of chemicals as well as their carcinogenic potential. The spectrum of toxicologic information sought on selected chemicals has been broadened by the multidisciplinary approach to evaluating chemicals. This paper describes the scientific rationale and experimental processes used by NTP in designing studies. Also, an outline of current NTP protocols are given for prechronic and chronic toxicity/carcinogenicity studies.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Haseman J. K., Huff J. E., Rao G. N., Arnold J. E., Boorman G. A., McConnell E. E. Neoplasms observed in untreated and corn oil gavage control groups of F344/N rats and (C57BL/6N X C3H/HeN)F1 (B6C3F1) mice. J Natl Cancer Inst. 1985 Nov;75(5):975–984. doi: 10.1093/jnci/75.5.975. [DOI] [PubMed] [Google Scholar]
- Haseman J. K., Huff J. E. Species correlation in long-term carcinogenicity studies. Cancer Lett. 1987 Oct 30;37(2):125–132. doi: 10.1016/0304-3835(87)90154-6. [DOI] [PubMed] [Google Scholar]
- Haseman J. K., Huff J., Boorman G. A. Use of historical control data in carcinogenicity studies in rodents. Toxicol Pathol. 1984;12(2):126–135. doi: 10.1177/019262338401200203. [DOI] [PubMed] [Google Scholar]
- Haseman J. K. Issues in carcinogenicity testing: dose selection. Fundam Appl Toxicol. 1985 Feb;5(1):66–78. doi: 10.1016/0272-0590(85)90050-8. [DOI] [PubMed] [Google Scholar]
- Haseman J. K. Statistical issues in the design, analysis and interpretation of animal carcinogenicity studies. Environ Health Perspect. 1984 Dec;58:385–392. doi: 10.1289/ehp.8458385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haseman J. K., Tharrington E. C., Huff J. E., McConnell E. E. Comparison of site-specific and overall tumor incidence analyses for 81 recent National Toxicology Program carcinogenicity studies. Regul Toxicol Pharmacol. 1986 Jun;6(2):155–170. doi: 10.1016/0273-2300(86)90031-0. [DOI] [PubMed] [Google Scholar]
- Luster M. I., Munson A. E., Thomas P. T., Holsapple M. P., Fenters J. D., White K. L., Jr, Lauer L. D., Germolec D. R., Rosenthal G. J., Dean J. H. Development of a testing battery to assess chemical-induced immunotoxicity: National Toxicology Program's guidelines for immunotoxicity evaluation in mice. Fundam Appl Toxicol. 1988 Jan;10(1):2–19. doi: 10.1016/0272-0590(88)90247-3. [DOI] [PubMed] [Google Scholar]
- Melnick R. L., Jameson C. W., Goehl T. J., Kuhn G. O. Application of microencapsulation for toxicology studies. I. Principles and stabilization of trichloroethylene in gelatin-sorbitol microcapsules. Fundam Appl Toxicol. 1987 May;8(4):425–431. doi: 10.1016/0272-0590(87)90128-x. [DOI] [PubMed] [Google Scholar]
- Melnick R. L., Jameson C. W., Goehl T. J., Maronpot R. R., Collins B. J., Greenwell A., Harrington F. W., Wilson R. E., Tomaszewski K. E., Agarwal D. K. Application of microencapsulation for toxicology studies. II. Toxicity of microencapsulated trichloroethylene in Fischer 344 rats. Fundam Appl Toxicol. 1987 May;8(4):432–442. doi: 10.1016/0272-0590(87)90129-1. [DOI] [PubMed] [Google Scholar]
- Portier C., Hoel D. Optimal design of the chronic animal bioassay. J Toxicol Environ Health. 1983 Jul;12(1):1–19. doi: 10.1080/15287398309530403. [DOI] [PubMed] [Google Scholar]
- Pryor G. T., Uyeno E. T., Tilson H. A., Mitchell C. L. Assessment of chemicals using a battery of neurobehavioral tests: a comparative study. Neurobehav Toxicol Teratol. 1983 Jan-Feb;5(1):91–117. [PubMed] [Google Scholar]
- Rall D. P., Hogan M. D., Huff J. E., Schwetz B. A., Tennant R. W. Alternatives to using human experience in assessing health risks. Annu Rev Public Health. 1987;8:355–385. doi: 10.1146/annurev.pu.08.050187.002035. [DOI] [PubMed] [Google Scholar]
- Rao G. N., Birnbaum L. S., Collins J. J., Tennant R. W., Skow L. C. Mouse strains for chemical carcinogenicity studies: overview of a workshop. Fundam Appl Toxicol. 1988 Apr;10(3):385–394. doi: 10.1016/0272-0590(88)90285-0. [DOI] [PubMed] [Google Scholar]
- Solleveld H. A., Haseman J. K., McConnell E. E. Natural history of body weight gain, survival, and neoplasia in the F344 rat. J Natl Cancer Inst. 1984 Apr;72(4):929–940. [PubMed] [Google Scholar]
- Tennant R. W., Margolin B. H., Shelby M. D., Zeiger E., Haseman J. K., Spalding J., Caspary W., Resnick M., Stasiewicz S., Anderson B. Prediction of chemical carcinogenicity in rodents from in vitro genetic toxicity assays. Science. 1987 May 22;236(4804):933–941. doi: 10.1126/science.3554512. [DOI] [PubMed] [Google Scholar]
- Tilson H. A. Behavioral indices of neurotoxicity: what can be measured? Neurotoxicol Teratol. 1987 Nov-Dec;9(6):427–443. doi: 10.1016/0892-0362(87)90055-9. [DOI] [PubMed] [Google Scholar]
- Weisburger E. K. History of the Bioassay Program of the National Cancer Institute. Prog Exp Tumor Res. 1983;26:187–201. doi: 10.1159/000407260. [DOI] [PubMed] [Google Scholar]
- Zeiger E. Carcinogenicity of mutagens: predictive capability of the Salmonella mutagenesis assay for rodent carcinogenicity. Cancer Res. 1987 Mar 1;47(5):1287–1296. [PubMed] [Google Scholar]


