Abstract
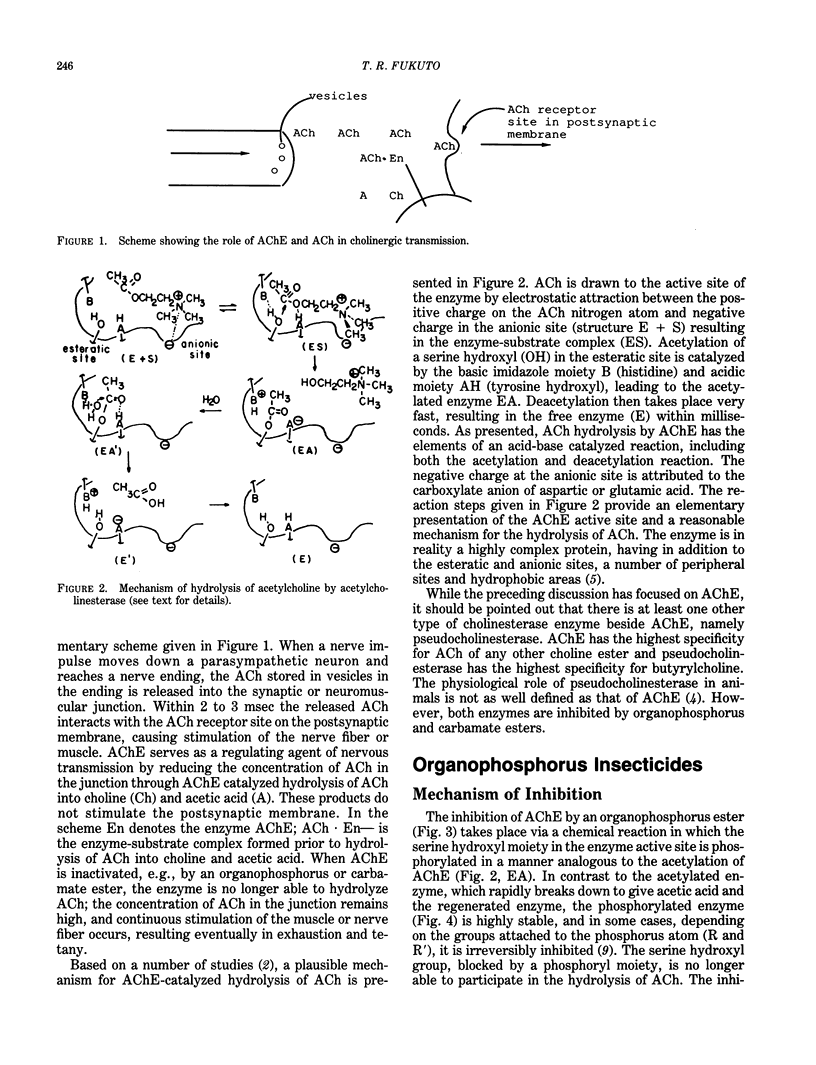
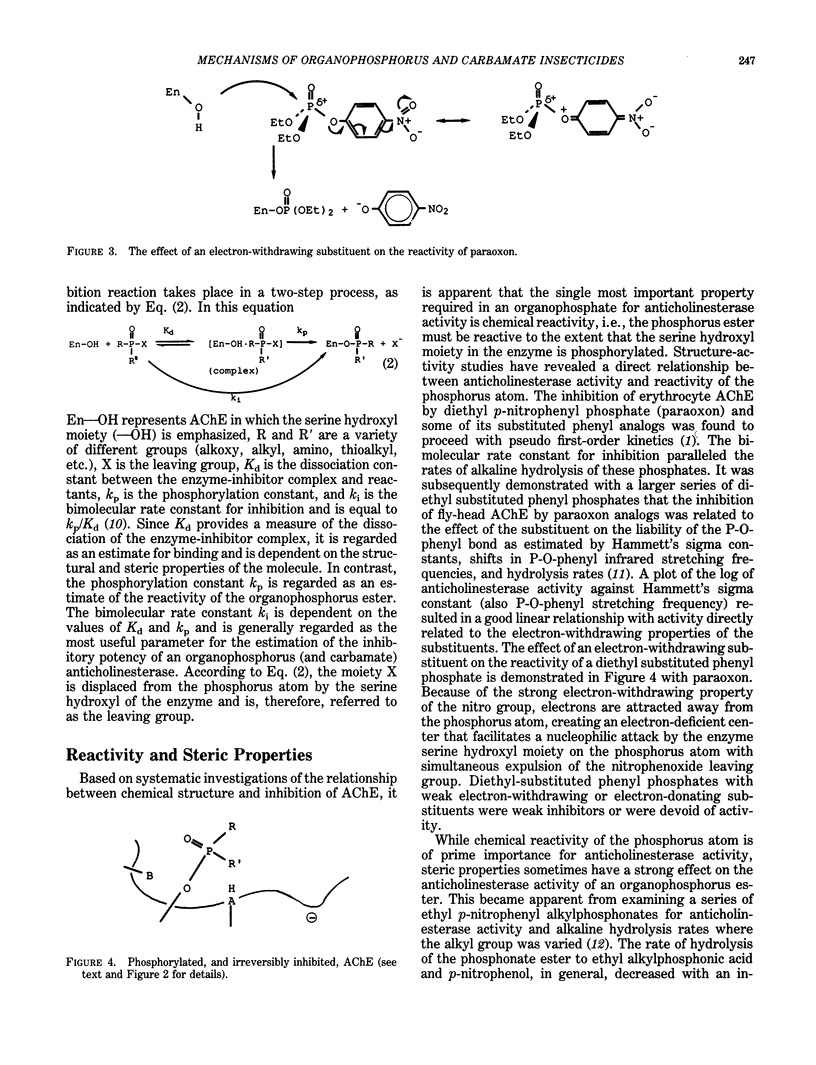
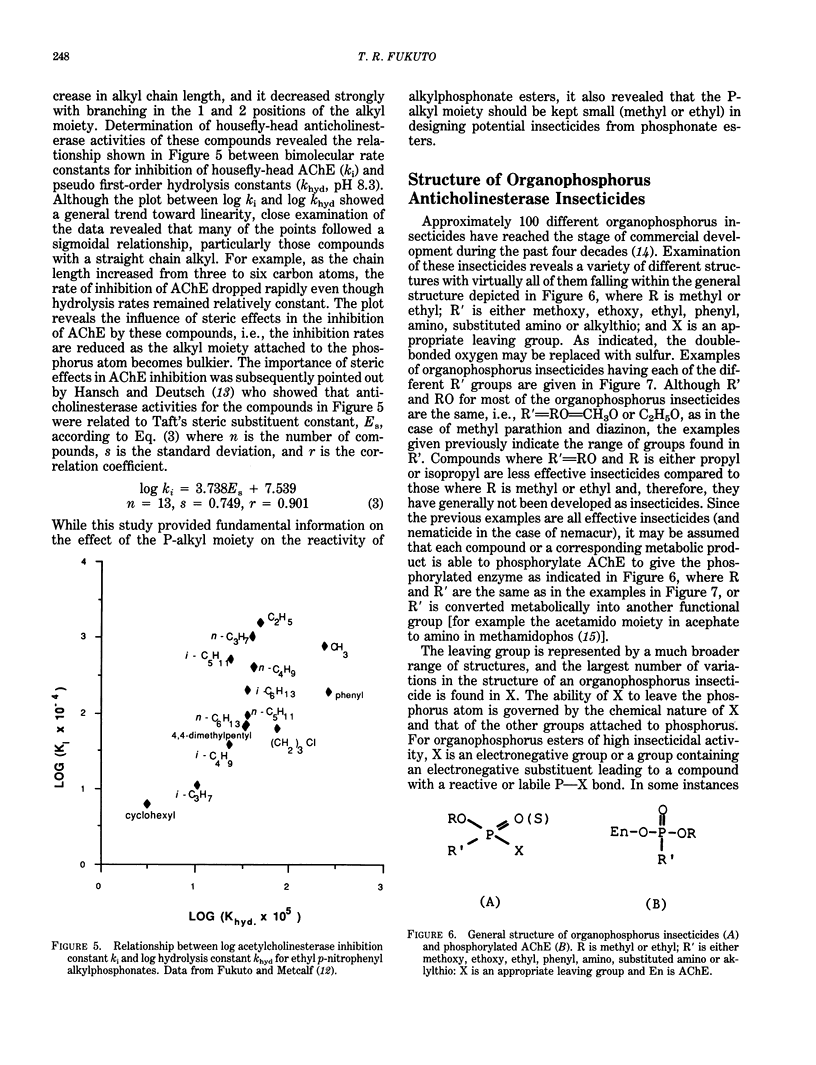
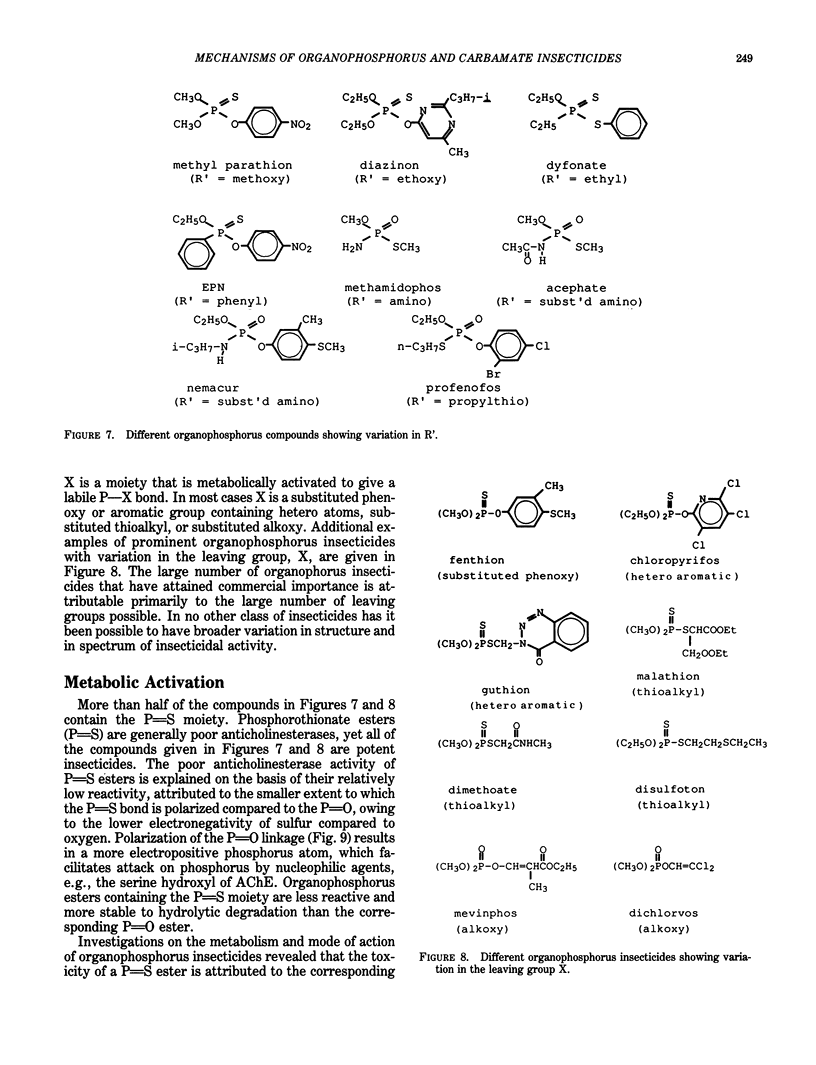
Organophosphorus and carbamate insecticides are toxic to insects and mammals by virtue of their ability to inactivate the enzyme acetylcholinesterase. This review addresses the mechanism of inhibition of acetylcholinesterase by organophosphorus and carbamate esters, focusing on structural requirements necessary for anticholinesterase activity. The inhibition of acetylcholinesterase by these compounds is discussed in terms of reactivity and steric effects. The role of metabolic activation or degradation in the overall intoxication process is also discussed.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALDRIDGE W. N., DAVISON A. N. The inhibition of erythrocyte cholinesterase by tri-esters of phosphoric acid. I. Diethyl p nitrophenyl phosphate (E600) and analogues. Biochem J. 1952 Apr;51(1):62–70. doi: 10.1042/bj0510062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ALDRIDGE W. N. Some properties of specific cholinesterase with particular reference to the mechanism of inhibition by diethyl p-nitrophenyl thiophosphate (E 605) and analogues. Biochem J. 1950 Apr;46(4):451–460. doi: 10.1042/bj0460451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dauterman W. C. Biological and nonbiological modifications of organophosphorus compounds. Bull World Health Organ. 1971;44(1-3):133–150. [PMC free article] [PubMed] [Google Scholar]
- GAGE J. C. A cholinesterase inhibitor derived from OO-diethyl O-p-nitrophenyl thiophosphate in vivo. Biochem J. 1953 Jun;54(3):426–430. doi: 10.1042/bj0540426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gnagey A. L., Forte M., Rosenberry T. L. Isolation and characterization of acetylcholinesterase from Drosophila. J Biol Chem. 1987 Sep 25;262(27):13290–13298. [PubMed] [Google Scholar]
- Hansch C., Deutsch E. W. The use of substituent constants in the study of structure-activity relationships in cholinesterase inhibitors. Biochim Biophys Acta. 1966 Sep 5;126(1):117–128. doi: 10.1016/0926-6585(66)90042-2. [DOI] [PubMed] [Google Scholar]
- MAIN A. R. AFFINITY AND PHOSPHORYLATION CONSTANTS FOR THE INHIBITION OF ESTERASES BY ORGANOPHOSPHATES. Science. 1964 May 22;144(3621):992–993. doi: 10.1126/science.144.3621.992. [DOI] [PubMed] [Google Scholar]
- Massoulié J., Bon S. The molecular forms of cholinesterase and acetylcholinesterase in vertebrates. Annu Rev Neurosci. 1982;5:57–106. doi: 10.1146/annurev.ne.05.030182.000421. [DOI] [PubMed] [Google Scholar]
- Metcalf R. L. Structure-activity relationships for insecticidal carbamates. Bull World Health Organ. 1971;44(1-3):43–78. [PMC free article] [PubMed] [Google Scholar]
- Ott P. Membrane acetylcholinesterases: purification, molecular properties and interactions with amphiphilic environments. Biochim Biophys Acta. 1985 Dec 9;822(3-4):375–392. doi: 10.1016/0304-4157(85)90016-4. [DOI] [PubMed] [Google Scholar]
- WILSON I. B., HATCH M. A., GINSBURG S. Carbamylation of acetvlcholinesterase. J Biol Chem. 1960 Aug;235:2312–2315. [PubMed] [Google Scholar]


