Abstract
Although the multilineage potential of human adipose-derived adult stromal cells (ADAS) has been well described, few published studies have investigated the biological and molecular mechanisms underlying osteogenic differentiation of mouse ADAS. We report here that significant osteogenesis, as determined by gene expression and histological analysis, is induced only when mouse ADAS are cultured in the presence of retinoic acid with or without recombinant human bone morphogenetic protein (BMP)-2 supplementation. Furthermore, a dynamic expression profile for the BMP receptor (BMPR) isoform IB was observed, with dramatic up-regulation during osteogenesis. Western blot analysis revealed that retinoic acid enhanced levels of BMPR-IB protein during the first 7 days of osteogenic differentiation and that RNAi-mediated suppression of BMPR-IB dramatically impaired the ability of ADAS to form bone in vitro. In contrast, absence of BMPR-IA did not significantly diminish ADAS osteogenesis. Our data therefore demonstrate that the osteogenic commitment of multipotent mouse ADAS requires retinoic acid, which enhances expression of the critical BMPR-IB isoform.
Keywords: adipogenesis, osteogenesis
Adipose-derived adult stromal cells (ADAS) are a readily isolated population of cells capable of differentiating down adipogenic, osteogenic, chondrogenic, myogenic, and possibly neurogenic lineages (1–3). The osteogenic potential of ADAS has been well described, with studies by Cowan et al. (4) and Hicok et al. (5) demonstrating the in vivo bone-forming capacity of ADAS. Autologous ADAS have also been used in combination with cancellous bone grafting to successfully repair a widespread calvarial defect after severe head injury in a 7-year-old child (6). Although these studies all suggest a high osteogenic potential for ADAS, the molecular mechanisms underlying ADAS differentiation toward osteogenic precursors and subsequent bone-forming osteoblasts remain unknown.
Several reports have demonstrated both retinoic acid (RA) and bone morphogenetic proteins (BMPs) to regulate osteogenesis or adipogenesis in a variety of cells (7, 8). RA has been shown to promote the differentiation of primary osteoblasts in vitro, resulting in enhanced expression of osteogenic genes and ultimate bone nodule deposition (8). In contrast, exogenous application of RA to 3T3-F442A cells or 3T3-L1 preadipocytes was found to inhibit adipogenic differentiation and lipid accumulation (9). Similar to RA, BMP-2 is also a potent inducer of bone formation (10). Interestingly, BMP-2 alone was found to enhance both markers for osteogenic and adipogenic differentiation in bipotential 3T3 preadipocytes (7, 11). In the presence of insulin, however, BMP-2 mildly inhibited adipogenesis of 3T3-F442A cells, resulting in decreased accumulation of lipid (7). These results suggest that both RA and BMP-2 may individually possess the ability to promote osteogenesis while inhibiting adipogenesis in cells with the ability to form either bone or fat. Studies by Skillington et al. (7) have actually demonstrated a collaboration of these factors to promote bone formation, highlighting a potential convergence in the regulation of mesenchymal cell osteogenic differentiation at the expense of adipogenesis.
Recent evidence has now allowed for a refinement in our understanding of BMP signaling with respect to skeletogenesis and adipogenesis (12, 13). By inducing expression of a constitutively active BMP receptor (BMPR) IB isoform in clonal 2T3 cells with the capacity to form both bone and fat, mineralized bone matrix could be induced without the addition of exogenous factors (14). In contrast, suppression of signaling through BMPR-IB was shown to dramatically impair bone formation and instead resulted in enhanced adipocyte differentiation (14). Interestingly, whereas the inhibition of BMPR-IA signaling eliminated the ability of 2T3 cells to form fat, increased bone nodule deposition was appreciated (14). Therefore, although BMPR-IA may play an important role in adipocyte differentiation, BMPR-IB may be more critical to the osteogenic commitment of these bipotent cells.
In combination with RA, specific BMP signaling may therefore assist in guiding cells along specific fates. Such a convergence between RA and BMP signaling may also be critical for redirecting ADAS away from an adipogenic fate and toward an osteogenic fate. In our study we explored this concept in detail and demonstrated that osteogenic differentiation of ADAS requires the presence of BMPR-IB.
Results
ADAS Are Capable of Adipogenic but Not Osteogenic Differentiation in Vitro.
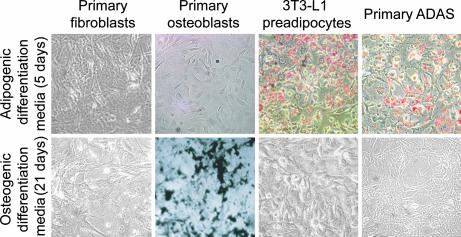
To determine ADAS differentiation potential in vitro, cells were cultured in standard adipogenic (5 days) or osteogenic (21 days) differentiation media (ODM). Cells were then stained for either lipid with Oil Red O (ORO) or mineralized extracellular matrix with von Kossa (Fig. 1). No adipogenic or osteogenic differentiation was observed with primary fibroblasts, and only appropriate lineage-specific differentiation was observed in 3T3-L1 preadipocytes and primary osteoblasts. Interestingly, although primary ADAS were capable of adipogenic differentiation, no extracellular matrix mineralization was observed with these cells. Mouse-derived ADAS were thus incapable of osteogenic differentiation in ODM used to induce in vitro bone formation in primary mouse osteoblasts and ADAS from humans and rats (1, 2, 15).
Fig. 1.
Histologic and gene analysis demonstrates ADAS differentiation capacity. ORO (Upper) and von Kossa (Lower) staining of multiple cell types after adipogenic and osteogenic differentiation. (Magnification: ×10.) Although primary fibroblasts demonstrated neither ORO nor von Kossa staining, primary osteoblasts and 3T3-L1 preadipocytes demonstrated lineage-appropriate staining. Note that primary ADAS were only capable of adipogenic differentiation.
The lack of osteogenesis was further confirmed by gene analysis after 21 days in ODM, examining expression patterns for the osteogenic differentiation markers runx2/cbfa1 (Runx2), osteopontin (Opn), and osteocalcin (Ocn) (Fig. 7A, which is published as supporting information on the PNAS web site). No significant induction in any of these genes relative to undifferentiated cells was appreciated, consistent with the observed failure of ADAS to undergo osteogenesis. In contrast, ADAS cultured in adipogenic differentiation media demonstrated significant up-regulation of PPARγ and adipsin (Fig. 7B, ∗, P < 0.05 for both).
The RA and BMP Signaling Pathways Are Present in Freshly Isolated ADAS.
Because studies have suggested a convergence of RA and BMP signaling to promote osteogenesis, the presence of each signaling pathway was evaluated in ADAS. Seen in Fig. 8A, which is published as supporting information on the PNAS web site, these cells elaborate transcripts for all six RA receptor isoforms (RARα, RARβ, RARγ, RXRα, RXRβ, and RXRγ). BMPR-IA, BMPR-IB, and BMPR-II expression were also identified in freshly isolated ADAS (Fig. 8B). Finally, RT-PCR demonstrated the presence of Smad1 and Smad5 transcripts, both effectors of the BMP signaling pathway in freshly isolated ADAS (Fig. 8C). Together, these data demonstrate that components of both RA and BMP signaling pathways are expressed in mouse ADAS.
RA Inhibits both ADAS Adipogenic Differentiation and Proliferation.
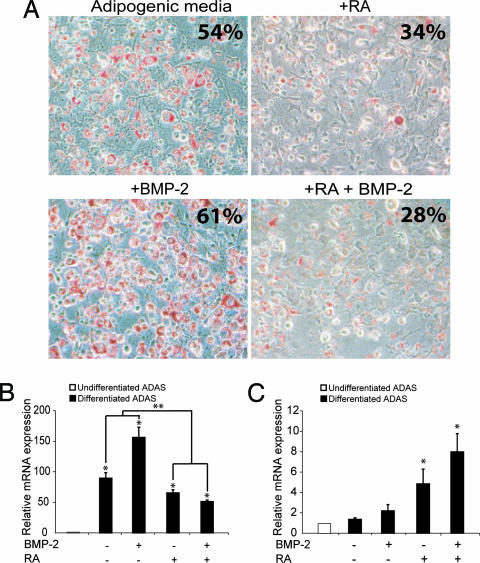
Given the reciprocal relationship between adipogenesis and osteogenesis, the effect of proosteogenic agents RA and BMP-2 were examined with respect to ADAS adipogenic differentiation. ADAS were cultured in standard adipogenic media supplemented with 2.5 μM RA and/or 50 ng/ml recombinant human (rh) BMP-2. Differentiation was assessed at 5 days with ORO staining and quantitative real-time RT-PCR (QRT-PCR) analysis for adipsin. As seen in Fig. 2A, treatment with rhBMP-2 slightly enhanced adipogenesis, whereas treatment with RA alone or RA in combination with rhBMP-2 markedly inhibited adipogenesis. QRT-PCR analysis of adipsin expression was consistent with the observed staining, demonstrating an 80-fold increase in transcript levels when cultured in adipogenic media (no RA or BMP-2) relative to cells cultured in control (growth) media (Fig. 2B, ∗, P < 0.05). Interestingly, this enhanced transcript expression was further increased by the addition of rhBMP-2 (140-fold; Fig. 2B, ∗, P < 0.05). Of note, adipogenic media supplemented with RA alone resulted in a significantly blunted adipsin up-regulation relative to adipogenic media alone or adipogenic media supplemented with rhBMP-2 (Fig. 2B, ∗∗, P < 0.05). Furthermore, combining rhBMP-2 and RA resulted in an even greater impairment of adipsin up-regulation relative to cells cultured in plain adipogenic media. Simultaneous evaluation of tissue nonspecific alkaline phosphatase (alk phos) expression demonstrated a reciprocal rise in response to RA or RA with rhBMP-2 (Fig. 2B, both ∗, P < 0.05), whereas rhBMP-2 alone led to no significant change relative to cells cultured in growth media (Fig. 2C). These data demonstrate that RA, either alone or in combination with rhBMP-2, inhibits ADAS adipogenic differentiation and may potentially induce osteogenic differentiation in vitro.
Fig. 2.
RA inhibits ADAS adipogenesis. (A) ORO staining for ADAS cultured in adipogenic media supplemented with RA and/or BMP-2. Note the dramatic reduction in ORO staining with the addition of RA or RA and BMP-2. Quantification of staining is noted in the upper right corner of each panel. (B) QRT-PCR analysis revealed an increase in adipsin expression for all conditions (∗, P < 0.05; n = 3 for each differentiated ADAS sample). However, a significant decrease in adipsin mRNA transcripts was appreciated with either RA or RA and BMP-2 supplementation relative to plain adipogenic media or adipogenic media with BMP-2 alone (∗∗, P < 0.05). (C) In contrast, alk phos was observed to be significantly up-regulated with RA or RA and BMP-2 (∗, P < 0.05; n = 3 for each differentiated ADAS sample).
The effect of RA or rhBMP-2 on the proliferation rate of ADAS was next determined by cell counting. After 7 days of culture in growth media or growth media supplemented with rhBMP-2 alone, an 8- to 9-fold increase in cell number was observed (Fig. 9, which is published as supporting information on the PNAS web site). In contrast, only a 2- to 3-fold increase in cell number was appreciated for cells cultured in growth media supplemented with either RA alone or RA with rhBMP-2 (Fig. 9, both ∗, P < 0.05). These data suggest that RA may direct ADAS from a more proliferative state toward a more differentiated state. And because RA has been shown to inhibit adipogenesis while simultaneously heightening alk phos expression, an alternative cell fate for ADAS may thus be promoted.
RA Promotes ADAS Osteogenic Differentiation.
With RA suppression of ADAS adipogenesis, osteogenic differentiation was subsequently evaluated. mRNA was harvested after 7, 14, or 21 days from cells cultured in osteogenic media supplemented with 2.5 μM RA and/or 50 ng/ml rhBMP-2. Early, middle, and late markers for osteogenic differentiation were assessed by QRT-PCR (Fig. 3A). Significant induction of Runx2 was seen in ADAS cultured in the presence of RA after 14 days (Fig. 3A Top, ∗, P < 0.05). Expression of Opn was also increased by RA exposure in a time-dependent fashion, with the greatest differential up-regulation observed at 21 days (Fig. 3A Middle, ∗, P < 0.05). Interestingly, increase in Ocn expression was seen in ADAS treated with either rhBMP-2 or RA after 14 days. However, the most dramatic up-regulation of Ocn was observed at 21 days in cells treated with RA alone or RA with rhBMP-2 (Fig. 3A Bottom, ∗, P < 0.05).
Fig. 3.
RA promotes ADAS osteogenesis. (A) QRT-PCR analysis revealed a statistically significant increase in Runx2 expression at day 14 when ADAS were cultured in ODM supplemented with either RA or RA and BMP-2. Opn and Ocn were also observed to increase in the presence of RA or RA and BMP-2, with the most dramatic differences noted at day 21 (n = 3 for each sample). (B) Minimal alkaline phosphatase and von Kossa staining was observed with ODM or ODM with BMP-2 supplementation alone. In contrast, significant staining was observed with RA or RA and BMP-2. Quantification of staining is noted in the upper right corner of each well.
To confirm that observed induction of genes was associated with osteoblastic differentiation of ADAS, histological assays were performed for both early and late (terminal) bone differentiation. Consistent with the expression pattern observed for Runx2, Opn, and Ocn, only ADAS cultured in the presence of RA stained strongly for alkaline phosphatase activity and bone nodule deposition; quantification of staining confirmed these findings (Fig. 3B). In addition, our data showed a cooperative effect of RA and BMP-2 on the level of alkaline phosphatase activity, extracellular matrix mineralization, and expression of osteogenic markers (Fig. 3). These effects by RA on osteogenesis were specific to cells with osteogenic potential. Although 3T3-L1 preadipocytes cultured in ODM with RA and rhBMP-2 supplementation also demonstrated staining for alkaline phosphatase, no bone nodule deposition was appreciated. Primary fibroblasts demonstrated minimal alkaline phosphatase staining and no staining for mineralized extracellular matrix (Fig. 10, which is published as supporting information on the PNAS web site).
BMPR Isoforms Are Differentially Expressed.
With apparent synergy between RA and BMP signaling in the osteogenic differentiation of ADAS (Fig. 3) and the ability of RA alone to induce extracellular matrix mineralization, we next examined the expression profiles for specific BMPRs. Relative transcript levels for all three BMPR isoforms were evaluated during both osteogenic differentiation with 2.5 μM RA and 50 ng/ml rhBMP-2 for 7 days and adipogenic differentiation for 5 days. Although only minor changes were noted in BMPR-IA and BMPR-II, marked alterations in the level of BMPR-IB were observed. A >700% increase in BMPR-IB transcripts was seen during osteogenic differentiation (Fig. 4A Left, ∗, P < 0.05), whereas a 90% decrease was observed during adipogenic differentiation (Fig. 4A Right, ∗, P < 0.05). To confirm that BMPR-IB was increased during osteoblastic differentiation, membrane fraction Western blot analysis was performed on ADAS cultured for 7 days in ODM supplemented with RA and/or rhBMP-2. Interestingly, cells cultured with RA or RA with rhBMP-2 demonstrated enhanced levels of BMPR-IB protein relative to cells cultured in ODM (Fig. 4B). Although a slight increase in BMPR-IB protein was also detected in response to rhBMP-2 alone, this effect was limited relative to that noted with RA or RA with rhBMP-2. Coomassie blue staining of the gel confirmed equivalent loading of total protein in each lane (Fig. 4C). These data suggest that signaling through BMPR-IB may be important for osteogenic differentiation of ADAS and that RA may effect such differentiation, at least in part, by markedly enhancing expression of BMPR-IB.
Fig. 4.
BMPR-IB expression is dynamic during ADAS osteogenesis and adipogenesis. (A) BMPR-IB transcripts were significantly up-regulated in ADAS undergoing osteogenic differentiation, whereas adipogenesis resulted in a reciprocal decline in BMPR-IB expression (∗, P < 0.05; n = 3 for each sample). In contrast, changes in the expression profile for BMPR-IA and BMPR-II were less dramatic. (B) Western blot analysis revealed that BMPR-IB in undifferentiated ADAS was enhanced at day 7 in the presence of RA or RA with BMP-2 relative to cells cultured in ODM or ODM supplemented with rhBMP-2 alone. (C) Coomassie blue staining demonstrated equivalent loading of protein in each lane.
BMPR-IB Suppression by RNAi.
Considering the observed 700% increase in BMPR-IB expression during osteogenic differentiation and the reciprocal 90% decrease during adipogenic differentiation, BMPR-IB may be potentially critical for guiding ADAS osteogenesis. To determine whether this receptor isoform was indeed required for bone formation, RNAi was used to suppress BMPR-IB levels in ADAS. QRT-PCR analysis for BMPR-IB transcript expression demonstrated variable efficacy for gene suppression induced by each of the five siRNA constructs designed relative to native, unperturbed ADAS (Fig. 5A, ∗, P < 0.05 for siRNA 1, 2, 3, and 5). In contrast, ADAS infected with a control siRNA construct or undergoing sham infection were found to express similar levels of BMPR-IB transcript relative to native, unperturbed cells. Western blot analysis demonstrated the presence of BMPR-IB protein in the membrane fraction of sham-infected and negative control siRNA-expressing ADAS (Fig. 5B). In addition, BMPR-IB could be detected in ADAS expressing siRNA constructs 2, 3, and 4. However, notable levels of protein could not be detected in ADAS infected with siRNA constructs 1 or 5, the two constructs that showed the greatest transcript suppression for BMPR-IB. Immunofluorescent staining provided further verification for QRT-PCR and Western blot analysis, demonstrating the absence of BMPR-IB in ADAS expressing siRNA constructs 1 or 5 (Fig. 5C).
Fig. 5.
RNAi-mediated suppression of BMPR-IB. (A) QRT-PCR analysis revealed that the most dramatic BMPR-IB transcript suppression occurred with siRNA constructs 1 and 5 (n = 3 for each sample). (B) Validation by Western blot confirmed the capacity for both of these constructs (1 and 5) to suppress BMPR-IB protein levels. (C) Immunofluorescent staining for BMPR-IB (green) was in accordance with QRT-PCR and Western blot data, showing minimal protein in ADAS expressing siRNA construct 1 or construct 5. Hoechst counterstaining (blue) can be appreciated in all groups.
Absence of BMPR-IB Impairs ADAS Osteogenic Differentiation.
von Kossa (data not shown) and Alizarin Red staining after 21 days of culture in ODM with 2.5 μM RA and 50 ng/ml rhBMP-2 revealed mineralized extracellular matrix deposition for control ADAS continuing to express BMPR-IB (Fig. 6A). In marked contrast, ADAS with RNAi-mediated down-regulation of BMPR-IB (siRNA 1 or 5) demonstrated a dramatic reduction in Alizarin Red staining (Fig. 6A). Spectrophotometric quantification confirmed these observations, with significantly less absorbance at 450 nm noted for ADAS expressing siRNA 1 or 5 (Fig. 6B, ∗, P < 0.05 for both).
Fig. 6.
Down-regulation of BMPR-IB impairs ADAS osteogenesis. (A) Alizarin Red staining revealed reduced mineral deposition in ADAS when BMPR-IB was suppressed by RNAi (siRNA 1 or 5). (B–D) Quantification demonstrated a significant reduction in the amount of Alizarin Red staining in these ADAS with down-regulated levels of BMPR-IB protein (∗, P < 0.05; n = 3 for each group). (B) In contrast, Cre-mediated disruption of BMPR-IA did not significantly impair osteogenesis. Despite significant suppression of intact BMPR-IA transcript with Adeno-CMV-Cre (∗, P < 0.01; n = 3 for each sample) (C), no appreciable change in Alizarin Red staining was observed (D). (E) Quantification revealed no difference in extracellular matrix mineralization in the absence of BMPR-IA (P > 0.05; n = 3 for each group).
ADAS Osteogenesis Proceeds Without BMPR-IA.
Although ADAS osteogenesis was found to be impaired by suppression of BMPR-IB, the contribution of BMPR-IA to ADAS bone formation was also evaluated by using cells harvested from conditional null mice containing the BMPR-IA allele flanked by loxP sites. ADAS infected with Adeno-CMV-Cre demonstrated a 90.2% reduction in intact BMPR-IA transcript relative to uninfected control cells (Fig. 6C, ∗, P < 0.01). Subsequent osteogenic differentiation of these cells in ODM with 2.5 μM RA and 50 ng/ml rhBMP-2 resulted in no appreciable reduction in staining for extracellular matrix mineralization when BMPR-IA was disrupted, as determined by Alizarin Red staining (Fig. 6D). Spectrophotometric quantification revealed no statistical difference when these transgenic ADAS treated with Adeno-CMV-Cre were compared with control cells or cells exposed to an empty adenovirus (P > 0.05) (Fig. 6E). Therefore, in contrast to BMPR-IB, disruption of BMPR-IA resulted in no appreciable inhibition of ADAS osteogenesis.
Discussion
Elucidating factors involved in the regulation of cell fate is critical to our understanding of development and postnatal repair. In this study we used knowledge garnered from investigations on cell lines and extended these findings to a primary, heterogeneous, multipotent cell population (7, 14). To perform our experiments we used the mouse inguinal fat pad as a source for ADAS. This source of ADAS avoids the limitations of a transformed cell line and has strong clinical relevance because it mimics the situation where human s.c. fat would be used. Our data established that signaling via all-trans RA induces osteogenic differentiation while simultaneously inhibiting adipogenic differentiation in murine ADAS. Such effects were enhanced by the addition of BMP-2. Furthermore, RA appeared to promote expression of BMPR-IB, thereby proposing a mechanism for the observed synergism between RA and BMP (7). Although other reports have also evaluated dexamethasone and vitamin D3, only RA and BMP signaling were investigated in this study to focus on minimal supplementation that would yield consistent results (2, 16, 17).
Although RA and BMP-2 are both known to be potent factors for osteoinduction, our study demonstrates that only RA has the ability to induce ADAS osteogenesis. Similar to reports on 3T3-F442A cells, BMP-2 alone was observed to actually enhance adipogenesis while failing to promote bone formation in mouse ADAS (7). The proosteogenic effect of RA is further punctuated by investigations demonstrating strong inhibition of adipocyte differentiation achieved through RA-mediated down-regulation of CCAAT/enhancer-binding protein β, a transcription factor known to stimulate adipogenesis (18, 19). Importantly, human ADAS have been shown to undergo osteogenic differentiation without the need for RA, suggesting potential interspecies differences between these cells. Despite this finding, several reports have demonstrated RA to both suppress human bone marrow mesenchymal cell proliferation and promote osteogenic differentiation of monkey embryonic stem cells, thus highlighting the potential breadth of implications for our findings on mouse-derived ADAS (20, 21).
Interestingly, although RA was found to be necessary for in vitro osteogenesis, in vivo osteogenesis by mouse ADAS has been shown to proceed without the need for exogenous growth factors. Regeneration of critical-sized calvarial defects has been demonstrated after the implantation of freshly harvested mouse ADAS seeded onto apatite-coated polylactic coglycolic acid scaffolds (4). The presence of a far more optimal environment for osteogenesis, however, likely contributed to this observed discrepancy. With an osteoconductive, osteoinductive scaffold and calvarial defect site together forming a fertile bed for bone formation, it is not entirely unexpected that RA would be unnecessary in this situation.
Our results suggest that a close relationship exists between ADAS adipogenesis and osteogenesis and that increased commitment toward bone formation occurs with a concomitant decrease in adipogenic capacity. Such an osteogenic commitment in mouse ADAS was best observed in response to RA and rhBMP-2. When used together, RA and rhBMP-2 increased both alkaline phosphatase activity and terminal osteoblast differentiation. Although Skillington et al. (7) have suggested an important cooperative interplay between RA and BMP signaling during the transdifferentiation of a committed preadipocyte cell line toward an osteogenic lineage. We have provided direct evidence of a convergence between RA and BMP signaling pathways in a primary cell type capable of both adipogenesis and osteogenesis.
Accumulating evidence suggests that specific signaling through either BMPR-IA or BMPR-IB has divergent effects on the process of skeletogenesis both in vitro and in vivo (12, 13, 22). In particular, studies using cells with multilineage potential have suggested that signaling through BMPR-IB regulates osteogenic differentiation (possibly via an interaction with RA) whereas signaling through BMPR-IA promotes adipogenesis (14). Paralleling these findings, our data confirmed the specific involvement of BMPR-IB in the process of mouse ADAS osteogenesis. QRT-PCR analysis showed a significant increase in the expression level of BMPR-IB during osteogenesis, whereas BMPR-IA and BMPR-II transcripts remained relatively stable. In contrast, a significant decrease in BMPR-IB was noted during adipogenesis. The dynamic expression profile for this isoform and the relatively static expression pattern for BMPR-IA and BMPR-II support the notion of BMPR-IB’s significance for osteogenic differentiation of mouse ADAS. Further highlighting this point, Western blot analysis of BMPR-IB during osteogenesis revealed that the protein level was directly and specifically related to RA treatment. And, in the absence of BMPR-IB, the osteogenic capacity of mouse ADAS was completely abolished, even in the presence of RA.
In contrast to BMPR-IB, we found the receptor isoform BMPR-IA to be less crucial for the osteogenic differentiation of mouse ADAS. Consistent with other published results, disruption of BMPR-IA through Cre-mediated DNA recombination did not significantly impair induction of extracellular matrix mineralization by our ADAS. Similar findings were noted by Chen et al. (14) using a truncated form of the BMPR-IA receptor in multipotent 2T3 cells. And, in BMPR-IA-deficient mice, normal formation of cartilaginous elements has been observed, suggesting no impairment in the differentiation of osteochondro-progenitors in the absence of BMPR-IA (23). Interestingly, other reports have demonstrated reduced osteogenic differentiation by harvested bone marrow from transgenic conditional-null mice for this specific receptor isoform (13). Targeted suppression of BMPR-IA in these studies, however, occurred in postnatal terminally differentiated osteoblasts using Cre-recombinase ligated to an element upstream of osteocalcin (13). Because we disrupted BMPR-IA before the induction of osteogenesis and reduced the inherent complexities of in vivo biology, the discrepancies noted are altogether reasonable.
Finally, we have demonstrated the ability of RA to promote expression of BMPR-IB. Although the exact mechanism of RA-mediated enhancement remains unknown, evaluation of the BMPR-IB promoter has not revealed any of the known classical RA response elements (data not shown). Therefore, it may be likely that indirect regulation of BMPR-IB resulted in the observations made. Other targets for RA have been suggested, including TAZ, a purported downstream rheostat regulating osteogenic and adipogenic differentiation in multipotent bone marrow mesenchymal cells (24). Interestingly, a 5′-AGGTCA-3′ DR1 RA response element has been noted upstream of the TAZ promoter, suggesting that this preferential binding site for RXR heterodimers may enhance transcription of this proosteogenic gene (25). Aside from these findings, several reports have also suggested alternative sites of RA and BMP convergence, including RA-mediated induction of Smad1 and Smad5 (26). Accordingly, osteogenesis may therefore be promoted while adipogenesis is inhibited. Such observations suggest that RA and BMP-2 signaling may converge at multiple levels. Furthermore, this convergence may very well depend on specific cell types and differentiation lineages.
The multilineage potential of ADAS, in combination with their ease of harvest, plentiful number, and ability to expand rapidly in culture, highlights the promise for ADAS to serve as an autogenous cell source that can be used to enhance the body’s endogenous ability to heal. For the repair and regeneration of skeletal defects, understanding the osteogenic capacity of ADAS is crucial for future clinical therapeutic use. Elucidating the mechanisms by which ADAS form bone would not only shed light on the process by which multipotent cell differentiation is controlled, but also help to direct the design of clinically applicable strategies in skeletal regenerative medicine.
Materials and Methods
Cell Harvest and Culture.
All experiments followed protocols approved by the Animal Facilities at Stanford University. 3T3-L1 cells were purchased from American Type Culture Collection (Manassas, VA). ADAS, osteoblast-enriched, and fibroblast cell cultures were established as previously described (4, 27).
Adipogenic and Osteogenic Differentiation.
First-passage cells were plated at a density of 2,500 cells per square centimeter for 24 h before the induction of differentiation. Adipogenic differentiation was induced by culturing cells for 3 days in adipogenic differentiation medium (DMEM/10% FBS/1% penicillin/streptomycin/10 μg/ml insulin/1 μM dexamethasone/0.5 mM methylxanthine/200 μM indomethacin), which was then changed to adipocyte maintenance medium (DMEM/10% FBS/1% penicillin/streptomycin/1 μg/ml insulin) for an additional 2 days before assessment of adipogenic differentiation. Osteogenic differentiation was induced by culturing ADAS in ODM (DMEM/10% FBS/1% penicillin/streptomycin/250 μM ascorbate-2-phosphate/10 mM β-glycerophosphate) with or without 2.5 μM all-trans RA (Sigma, St. Louis, MO) and/or 50 ng/ml rhBMP-2 (R & D Systems, Minneapolis, MN) supplementation for 7, 14, or 21 days (28).
Histological Analysis.
ORO, alkaline phosphatase, and bone nodule staining by von Kossa was performed as previously described (28). Extracellular matrix mineralization was evaluated by using Alizarin Red staining (29). All differentiation studies were performed in triplicate to facilitate statistical analysis. Digital images of each well in its entirety were acquired, and the area of staining was determined by using Scion Image Analysis (National Institutes of Health, Bethesda, MD). All wells were counterstained with hematoxylin to confirm uniform cellularity. Spectrophotometric quantification of Alizarin Red staining was performed as previously described (29).
Proliferation Assay.
To determine proliferation, first-passage ADAS were plated at 5,000 cells per well in a 12-well tissue culture plate. Cells were harvested every other day up to 7 days, and cell counting was performed (see Supporting Materials and Methods, which is published as supporting information on the PNAS web site).
RT-PCR and QRT-PCR.
RT-PCR was performed by using PCR Master Mix (Promega, Madison, WI) to evaluate for expression of RA and BMPRs. QRT-PCR with SYBR green detection was performed to determine expression levels for Runx2, alk phos, Opn, Ocn, PPARγ, adipsin, BMPR-IA, BMPR-IB, and BMPR-II (see Supporting Materials and Methods).
Creation and Validation of BMPR-IB siRNA Constructs.
Five individual siRNA constructs targeting BMPR-IB were identified with the assistance of siRNA Target Finder (Ambion, Austin, TX), and target gene specificity was confirmed by using NIH BLAST (Fig. 11, which is published as supporting information on the PNAS web site). Infection-competent retroviruses expressing each siRNA were generated as previously reported (30) (and described in Supporting Materials and Methods). ADAS were infected with different BMPR-IB-targeted siRNA constructs or with a negative control GFP-targeted siRNA (30). After puromycin selection (2 μg/ml), ADAS were harvested for RNA, and QRT-PCR analysis was performed to evaluate for levels of BMPR-IB transcript normalized to glyceraldehyde-3-phosphate dehydrogenase (24). Western blot analysis and immunofluorescent staining were performed for validation (see Supporting Materials and Methods). ADAS expressing BMPR-IB siRNA constructs were subsequently cultured in ODM with 2.5 μM RA and 50 ng/ml rhBMP-2 for 21 days before evaluation for extracellular matrix mineralization as previously described. In addition, sham-infected and control siRNA-infected ADAS also underwent osteogenic differentiation for comparison. All experiments were performed in triplicate.
Cre-Dependent Disruption of BMPR-IA.
ADAS were harvested from BMPR-IA conditional-null mice with exon 4 of the BMPR-IA allele flanked by loxP sites (13). Cells were then cultured in growth medium containing 50 multiplicities of infection of Cre-expressing adenovirus (Ad-CMV-Cre, Vector Biolabs, Philadelphia, PA) or empty adenovirus for 48 h. Evaluation of Cre-mediated DNA recombination for BMPR-IA disruption was performed by QRT-PCR using primers previously described (13). ADAS with or without BMPR-IA disruption were then induced to undergo osteogenic differentiation with ODM containing both 2.5 μM RA and 50 ng/ml rhBMP-2. Extracellular matrix mineralization was determined after 21 days by using Alizarin Red staining and compared with control ADAS from the same mice with no adenoviral infection.
Western Blot Analysis.
Smad protein levels were evaluated by whole-cell Western blot analysis. Membrane fraction protein isolation was performed for BMPR-IB Western blot analysis. Coomassie blue PhastGel (GE Healthcare, Piscataway, NJ) staining was used to confirm equivalent loading of protein in each lane (see Supporting Materials and Methods).
Statistical Analysis.
Statistical analyses were performed by using Student’s t test or one-way ANOVA between groups with Tukey’s multiple comparison test on Prism software (Prism version 3.0 for Windows, GraphPad, San Diego, CA). A P value <0.05 was considered statistically significant.
Supplementary Material
Acknowledgments
We thank Ali Salim and Monika Tataria for technical support and Matthew Kwan for editorial assistance. Floxed BMPR-IA mice were provided by Yuji Mishina (National Institutes of Health). This work was supported by National Institutes of Health Grants R01 DE 14526 and R01 DE 13194 (to M.T.L.) and the Oak Foundation (M.T.L.), a Howard Hughes Medical Institute Student Fellowship (to Y.-Y.S.), and an Ethicon–Society of University Surgeons Research Fellowship (to D.C.W.).
Glossary
Abbreviations
- rh
recombinant human
- RA
retinoic acid
- ADAS
adipose-derived adult stromal cell
- BMP
bone morphogenetic protein
- BMPR
BMP receptor
- QRT-PCR
quantitative real-time RT-PCR
- ORO
Oil Red O
- ODM
osteogenic differentiation medium.
Footnotes
Conflict of interest statement: No conflicts declared.
References
- 1.Zuk P. A., Zhu M., Ashjian P., De Ugarte D. A., Huang J. I., Mizuno H., Alfonso Z. C., Fraser J. K., Benhaim P., Hedrick M. H. Mol. Biol. Cell. 2002;13:4279–4295. doi: 10.1091/mbc.E02-02-0105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zuk P. A., Zhu M., Mizuno H., Huang J., Futrell J. W., Katz A. J., Benhaim P., Lorenz H. P., Hedrick M. H. Tissue Eng. 2001;7:211–228. doi: 10.1089/107632701300062859. [DOI] [PubMed] [Google Scholar]
- 3.Gimble J. M., Guilak F. Curr. Top. Dev. Biol. 2003;58:137–160. doi: 10.1016/s0070-2153(03)58005-x. [DOI] [PubMed] [Google Scholar]
- 4.Cowan C. M., Shi Y. Y., Aalami O. O., Chou Y. F., Mari C., Thomas R., Quarto N., Contag C. H., Wu B., Longaker M. T. Nat. Biotechnol. 2004;22:560–567. doi: 10.1038/nbt958. [DOI] [PubMed] [Google Scholar]
- 5.Hicok K. C., Du Laney T. V., Zhou Y. S., Halvorsen Y. D., Hitt D. C., Cooper L. F., Gimble J. M. Tissue Eng. 2004;10:371–380. doi: 10.1089/107632704323061735. [DOI] [PubMed] [Google Scholar]
- 6.Lendeckel S., Jodicke A., Christophis P., Heidinger K., Wolff J., Fraser J. K., Hedrick M. H., Berthold L., Howaldt H. P. J. Craniomaxillofac. Surg. 2004;32:370–373. doi: 10.1016/j.jcms.2004.06.002. [DOI] [PubMed] [Google Scholar]
- 7.Skillington J., Choy L., Derynck R. J. Cell Biol. 2002;159:135–146. doi: 10.1083/jcb.200204060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Song H. M., Nacamuli R. P., Xia W., Bari A. S., Shi Y. Y., Fang T. D., Longaker M. T. J. Cell. Physiol. 2005;202:255–262. doi: 10.1002/jcp.20115. [DOI] [PubMed] [Google Scholar]
- 9.Kuri-Harcuch W. Differentiation. 1982;23:164–169. doi: 10.1111/j.1432-0436.1982.tb01279.x. [DOI] [PubMed] [Google Scholar]
- 10.Chen D., Zhao M., Mundy G. R. Growth Factors. 2004;22:233–241. doi: 10.1080/08977190412331279890. [DOI] [PubMed] [Google Scholar]
- 11.Ji X., Chen D., Xu C., Harris S. E., Mundy G. R., Yoneda T. J. Bone Miner. Metab. 2000;18:132–139. doi: 10.1007/s007740050103. [DOI] [PubMed] [Google Scholar]
- 12.Ashique A. M., Fu K., Richman J. M. Int. J. Dev. Biol. 2002;46:243–253. [PubMed] [Google Scholar]
- 13.Mishina Y., Starbuck M. W., Gentile M. A., Fukuda T., Kasparcova V., Seedor J. G., Hanks M. C., Amling M., Pinero G. J., Harada S., Behringer R. R. J. Biol. Chem. 2004;279:27560–27566. doi: 10.1074/jbc.M404222200. [DOI] [PubMed] [Google Scholar]
- 14.Chen D., Ji X., Harris M. A., Feng J. Q., Karsenty G., Celeste A. J., Rosen V., Mundy G. R., Harris S. E. J. Cell Biol. 1998;142:295–305. doi: 10.1083/jcb.142.1.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huang J. I., Beanes S. R., Zhu M., Lorenz H. P., Hedrick M. H., Benhaim P. Plast. Reconstr. Surg. 2002;109:1033–1041. doi: 10.1097/00006534-200203000-00037. discussion 1042–1043. [DOI] [PubMed] [Google Scholar]
- 16.Hattori H., Sato M., Masuoka K., Ishihara M., Kikuchi T., Matsui T., Takase B., Ishizuka T., Kikuchi M., Fujikawa K. Cells Tissues Organs. 2004;178:2–12. doi: 10.1159/000081088. [DOI] [PubMed] [Google Scholar]
- 17.Shevde N. K., Plum L. A., Clagett-Dame M., Yamamoto H., Pike J. W., DeLuca H. F. Proc. Natl. Acad. Sci. USA. 2002;99:13487–13491. doi: 10.1073/pnas.202471299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yun Z., Maecker H. L., Johnson R. S., Giaccia A. J. Dev. Cell. 2002;2:331–341. doi: 10.1016/s1534-5807(02)00131-4. [DOI] [PubMed] [Google Scholar]
- 19.Schwarz E. J., Reginato M. J., Shao D., Krakow S. L., Lazar M. A. Mol. Cell. Biol. 1997;17:1552–1561. doi: 10.1128/mcb.17.3.1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Oliva A., Borriello A., Zeppetelli S., Di Feo A., Cortellazzi P., Ventriglia V., Criscuolo M., Zappia V., Della Ragione F. Mol. Cell. Biochem. 2003;247:55–60. doi: 10.1023/a:1024192719178. [DOI] [PubMed] [Google Scholar]
- 21.Yamashita A., Takada T., Narita J., Yamamoto G., Torii R. Cloning Stem Cells. 2005;7:232–237. doi: 10.1089/clo.2005.7.232. [DOI] [PubMed] [Google Scholar]
- 22.Kaps C., Hoffmann A., Zilberman Y., Pelled G., Haupl T., Sittinger M., Burmester G., Gazit D., Gross G. Biofactors. 2004;20:71–84. doi: 10.1002/biof.5520200202. [DOI] [PubMed] [Google Scholar]
- 23.Yoon B. S., Ovchinnikov D. A., Yoshii I., Mishina Y., Behringer R. R., Lyons K. M. Proc. Natl. Acad. Sci. USA. 2005;102:5062–5067. doi: 10.1073/pnas.0500031102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hong J. H., Hwang E. S., McManus M. T., Amsterdam A., Tian Y., Kalmukova R., Mueller E., Benjamin T., Spiegelman B. M., Sharp P. A., et al. Science. 2005;309:1074–1078. doi: 10.1126/science.1110955. [DOI] [PubMed] [Google Scholar]
- 25.Rastinejad F., Wagner T., Zhao Q., Khorasanizadeh S. EMBO J. 2000;19:1045–1054. doi: 10.1093/emboj/19.5.1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li X., Schwarz E. M., Zuscik M. J., Rosier R. N., Ionescu A. M., Puzas J. E., Drissi H., Sheu T. J., O’Keefe R. J. Endocrinology. 2003;144:2514–2523. doi: 10.1210/en.2002-220969. [DOI] [PubMed] [Google Scholar]
- 27.Kong W., Longaker M. T., Lorenz H. P. J. Biol. Chem. 2004;279:55334–55340. doi: 10.1074/jbc.M410804200. [DOI] [PubMed] [Google Scholar]
- 28.Shi Y. Y., Nacamuli R. P., Salim A., Longaker M. T. Plast. Reconstr. Surg. 2005;116:1686–1696. doi: 10.1097/01.prs.0000185606.03222.a9. [DOI] [PubMed] [Google Scholar]
- 29.Tataria M., Quarto N., Longaker M. T., Sylvester K. G. J. Pediatr. Surg. 2006;41:624–632. doi: 10.1016/j.jpedsurg.2005.12.001. [DOI] [PubMed] [Google Scholar]
- 30.Sen G., Wehrman T. S., Myers J. W., Blau H. M. Nat. Genet. 2004;36:183–189. doi: 10.1038/ng1288. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.