Abstract
Glycomics is emerging as a new field for the biology of complex glycoproteins and glycoconjugates. The lack of versatile glycan-labeling methods has presented a major obstacle to visualizing at the cellular level and studying glycoconjugates. To address this issue, we developed a fluorescent labeling technique based on the Cu(I)-catalyzed [3 + 2] cycloaddition, or click chemistry, which allows rapid, versatile, and specific covalent labeling of cellular glycans bearing azide groups. The method entails generating a fluorescent probe from a nonfluorescent precursor, 4-ethynyl-N-ethyl-1,8-naphthalimide, by clicking the fluorescent trigger, the alkyne at the 4 position, with an azido-modified sugar. Using this click-activated fluorescent probe, we demonstrate incorporation of an azido-containing fucose analog into glycoproteins via the fucose salvage pathway. Distinct fluorescent signals were observed by flow cytometry when cells treated with 6-azidofucose were labeled with the click-activated fluorogenic probe or biotinylated alkyne. The intracellular localization of fucosylated glycoconjugates was visualized by using fluorescence microscopy. This technique will allow dynamic imaging of cellular fucosylation and facilitate studies of fucosylated glycoproteins and glycolipids.
Keywords: alkynes, azides, cycloaddition, fluorescent probes, glycoconjugates
Glycosylation is an important posttranslational modification affecting >50% of eukaryotic proteins (1). In particular, fucosylation has been recognized as an important glycosylation event. l-Fucose is often a terminal sugar in glycans that participate in important cell–cell interactions and cell migration processes in connection with physiological and pathological processes such as fertilization, embryogenesis, lymphocyte trafficking, immune responses, and cancer metastasis (2–6). The investigation of fucosylated glycans in healthy and disease processes is therefore of great interest. However, synthesis, isolation, and characterization of glycans remain a major challenge, due mainly to their structural complexity (7, 8). Therefore, development of specific tagging methods to visualize and quantify glycoconjugates in situ has become a subject of keen interest (9–12).
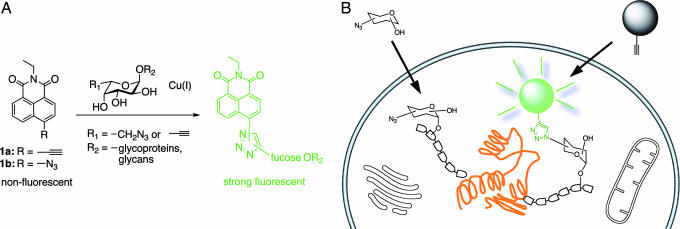
Proteins can be labeled fluorescently in a facile manner by genetically engineering fusion constructs with fluorescent labels, such as GFP. This technology offers the advantage of investigating proteomics via dynamic imaging of living cells (13). However, the same versatility is not available in the field of glycomics, because glycosylation is a nontemplated posttranslational process. Thus, new and facile chemical approaches are required to address glycomics. Here, we report two click-activated fluorescent probes based on 1,8-naphthalimide, which can selectively label azido- or acetylene-modified l-fucose analogs by a Cu(I)-catalyzed azide-alkyne ligation, which triggers their fluorescence (Fig. 1A). In addition, we demonstrate that this click-activated fluorogenic labeling technique is sufficiently sensitive and selective to visualize fucosylated glycoproteins in whole cells (Fig. 1B).
Fig. 1.
General strategy for glycan labeling. (A) Probe structures based on 1,8-naphthalimide include an azide or alkyne at a position of the ring that will allow a fluorogenic ligation with 6-modified fucose analogs. The fluorescence adduct is generated when probes are reacted with the azido/alkynyl group of fucosides via Cu(I)-catalyzed [3 + 2] cycloaddition. (B) Strategy for specific fluorescent labeling of fucosylated glycans in cells. Covalent modification of the target glycan with probes 1a or 1b results in production of fluorescently labeled glycoproteins bearing modified fucose (azidofucose shown).
Recently, several elegant methods have been developed to label chemically modified sugars in glycoproteins by using a ketone-aminooxy/hydrazide ligation (14, 15), Staudinger ligation (16), and Michael addition (17). These methods entail multistep procedures that use secondary fluorescent dyes for visualization, which often causes high background noise that is difficult to remove from intracellular environments or tissues. An ideal alternative would be a fluorogenic reaction, where the ligated product generates a strong detectable signal, whereas unreacted reagent remains traceless. Our objective, therefore, focused on a bioorthogonal-labeling approach based on the Cu(I)-catalyzed [3 + 2] cycloaddition reaction (18, 19). Desirable features of this reaction include small, stable coupling partners (the azide and alkyne), fast reaction rates, and the formation of a triazole unit that modulates the fluorescent emission response of the probe via electron-donating properties. During our study, this click-activated approach also was used to control the fluorescent property of coumarins by other groups (20, 21). However, the coumarin system suffers from UV excitation and short wavelength fluorescence, which might increase the background signal in biological systems. Our fluorogenic probe design is based on 4-amino-1,8-naphthalimide, which absorbs light in the visible region and emits at long wavelengths (λmax 540–550 nm) (22). We postulated that the fluorescent signal of 1,8-naphthalimides might be modulated by the formation of a triazole ring, because substitutions at the 4 position with an electron-donating group are known to strongly affect their fluorescent properties (23). Two 1,8-naphthalimide derivatives were designed for click-activated fluorescent properties (1a and 1b) with either an azide or alkyne moiety attached at the 4 position. This design provides an opportunity to react the probe with a fucose analog having the corresponding azido or alkynyl functionality. The substrate tolerance of enzymes in the biosynthesis of fucosylated glycoconjugates can be used to determine which modified fucose should be implemented.
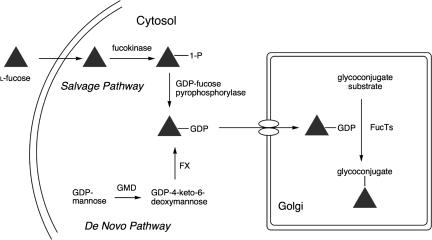
Two pathways, the de novo pathway and the salvage pathway, have been proposed in the synthesis of the fucose donor, GDP-fucose, which is used in the construction of fucosylated oligosaccharides (Fig. 2; ref. 24). The de novo pathway, which is believed to be the primary source of GDP-fucose, uses GDP-mannose as a starting point. The salvage pathway contributes additional GDP-fucose by the action of two enzymes, fucose kinase and GDP-fucose pyrophosphorylase, which act on fucose derived from extracellular and lysosomal sources (2, 25–27). It was speculated that azido and/or alkyne-modified fucose analogs might be incorporated into glycoproteins via the salvage pathway, if the small size and bioorthogonality of azido/alkynyl groups could be tolerated by the requisite enzymes (11, 28). Our design of azido/alkynyl functionalities at position 6 on fucose was prompted by reports that fucosyltransferases have broad tolerance for 6-substituents (29–31).
Fig. 2.
Biosynthetic pathways for GDP-fucose. The de novo pathway transforms GDP-mannose into GDP-fucose via two enzymes, GDP-mannose 4,6-dehydratase (GMD) and GDP-keto-6-deoxymannose 3,5-epimerase/4-reductase (FX protein). The salvage pathway utilizes free fucose in the cytosol to create GDP-fucose by the action of fucose kinase and GDP-fucose pyrophosphorylase. The resulting GDP-fucose is used by fucosyltransferases (FucTs) in the Golgi apparatus to catalyze transfer onto glycoconjugates.
Results
Synthesis of Fluorogenic Probes.
Compounds 1a and 1b were synthesized from 4-bromo-1,8-naphthalic anhydride (see supporting information, which is published as supporting information on the PNAS web site, for the synthesis of compounds). Their coupling partners, fucose analogs 2a and 2b, were prepared from l-galactose (32). Conversion from the 6-hydroxyl group into the alkyne was achieved by using the Seyferth/Gilbert reagent in excellent yield (33).
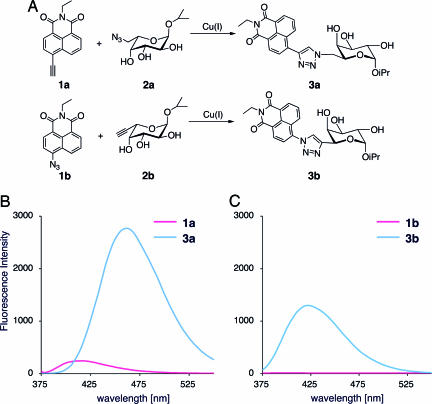
Reactivity and click-activated fluorogenic properties of compounds 1a and 1b were tested by performing a [3 + 2] cycloaddition with model fucose analogs 2a and 2b, respectively (Fig. 3). As expected, the ligation reactions produced in a significant increase in fluorescence intensity. The emission maximum of 3a was 462 nm with a quantum yield of 0.36 when excited at 357 nm. Compound 3b also showed strong emission with a maximum at 422 nm and a quantum yield of 0.29, whereas the parent compound 1b showed no fluorescence. Unfortunately, the reaction rate between alkyne probe 1a and azidofucose 2a was very slow. Although the reaction of azido probe 1b was completed in <30 min, only 10% of 1a was converted to 3a after 24 h by using the same conditions (monitored by LC-MS). The relatively low reactivity of 1a compared with 1b might be explained by the highly reactive aromatic azide on the latter (34). However, in the presence of the Tris(triazolyl)amine ligand, which is an effective Cu(I) catalyst (20, 35), the reaction between 1a and 2a was completed within 30 min.
Fig. 3.
The “click” reaction of probes 1a and 1b with fucose derivatives results in highly fluorescent adducts (A) and fluorescence spectra of compounds 1a and 1b and their click products 3a and 3b (B and C).
A fluorescence quenching technique was used to identify catalysts for the copper-catalyzed cycloaddition (36). However, this method has limitations because of a high false-positive rate caused by ligand itself or other side reactions. The slow reaction between 1a and 2a, however, is useful for catalyst screening because of its inherently low background signal. In such a screen, we performed the reaction between 1a and 2a in microtiter plates in the presence of individual amino acids. The effect of the ligand effect on the reaction rate was visualized by holding the 96-well microtiter plate under a long wavelength UV lamp (365 nm) and analyzed with a standard fluorescence multiplate reader (see supporting information). The assay identified histidine as the best amino acid catalyst for the Cu(I)-catalyzed 1,3-dipolar cycloaddition reaction. This experiment demonstrates that compound 1a is generally useful for rapid screening of novel catalysts for azide-alkyne cycloaddition reaction.
Fluorescent Labeling of Fucosylated Glycoprotein.
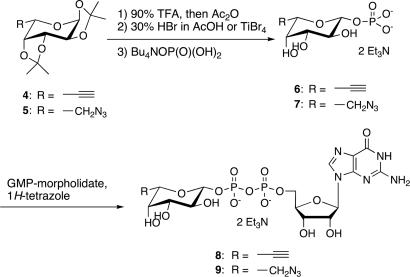
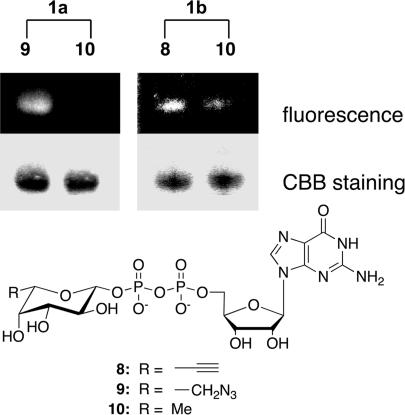
To demonstrate that the naphthalimide probes can be used to specifically label fucosylated glycoproteins in vitro, GDP-fucose derivatives 8 and 9, which contain either an acetylene or azido group at the 6 position of the fucose, were synthesized (Scheme 1). Briefly, fucose analogs 4 and 5 were converted to the corresponding 1-bromides, which then were substituted directly by a phosphate group. Notably, under standard bromination conditions, such as HBr/AcOH and bromotrimethylsilane, the azide was substituted with bromide. Therefore, TiBr4 was used instead, as described by Srivastava (30). The fucose-1-phosphates were converted successfully to the corresponding GDP-fucose analogs by using 1H-tetrazole as a catalyst (37). The tolerability of GDP-fucose analogs 8 and 9 for human α-1,3-fucosyltranseferases (FucTs) II–VII was confirmed by using a standard microtiter plate assay (38, 39). They were then used to elaborate the glycoprotein substrate, human α1-acid glycoprotein (AGP), an acute-phase protein. As fucosylation of AGP is associated with inflammatory diseases, the degree of fucosylation of AGP could be a potential diagnostic or prognostic marker (40, 41). AGP was fucosylated by using FucT V in the presence of GDP-fucose analogs 8 or 9. After gel filtration, the fucosylated AGP was labeled by using the corresponding naphthalimide 1a or 1b for 4 h at room temperature. Labeled protein was analyzed by SDS/PAGE and visualized by UV light (365 nm). Both click-activated probes 1a and 1b showed clear fluorescent bands when AGP was fucosylated with analogs 9 and 8, respectively. No band was present when unmodified GDP-fucose 10 was used, indicating a specific ligation between probe 1a and the azidofucose analog transferred onto the protein glycan (Fig. 4). However, the azido probe 1b slowly decomposed into a strongly fluorescent 4-amino derivative, resulting in a slight background signal (see supporting information). Thus, the alkyne probe is more reliable for labeling.
Scheme 1.
Synthesis of GDP-fucose analogs.
Fig. 4.
Visualization of AGP after FucT transfer of modified GDP-fucose and labeling reaction with fluorogenic probes 1a and 1b. Treated protein was separated by SDS/PAGE and visualized by UV light (Top) and Coomassie blue staining (CBB) (Middle). (Bottom) Modified GDP-fucose.
Fluorescent Labeling of Fucosylated Glycoconjugates on the Cell Surface.
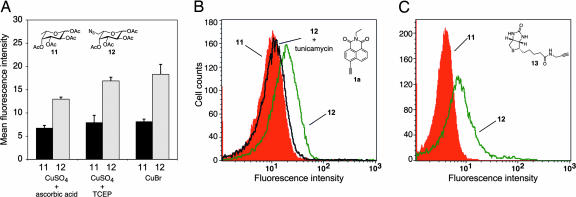
We next investigated the labeling of azide-tagged glycoproteins expressed on the cell surface by using the alkyne click-activated probe 1a. First, the effect of various Cu(I) sources on the copper-catalyzed cell-surface labeling reaction was investigated. Cu(I) is typically generated including in situ by the reduction of Cu(II) by conventional reducing agents or the direct addition of Cu(I) as a salt (42). To incorporate azidofucose into glycoproteins, we investigated both methods by using in situ reduction with ascorbic acid and Tris(carboxyethyl)phosphine (TCEP) and direct addition of CuBr to glycan samples. Jurkat cells were cultured in the presence of globally acetylated 6-azido-l-fucose 12 (acetylation was incorporated to increase uptake of the sugar; refs. 16 and 43) in an effort to incorporate the analog via the salvage pathway. After incubating for 3 days, the cells were labeled with 1a in the presence of Cu(I) sources, and the fluorescent intensities were measured by flow cytometry. The azidofucose-treated cells exhibited a distinct increase in fluorescence by using all three copper sources when compared with the cells treated with natural fucose (Fig. 5A). This result suggests that fluorescent increases result from specific fluorogenic modification of azido-glycans. The CuBr source produced the highest mean fluorescent intensity, in agreement with previous reports from Tirrell et al. (42). To exclude the possibility that the observed fluorescence was generated by free azidofucose, cells were treated with tunicamycin, an inhibitor of N-linked glycoprotein biosynthesis. Tunicamycin treatment shifted the entire population to lower fluorescence (Fig. 5B), indicating that most of the azidofucose was incorporated into N-linked glycoproteins via the biosynthetic pathway.
Fig. 5.
Analysis of fucosylated glycoproteins on the cell surface of Jurkat cells by flow cytometry. (A) Comparison of labeling efficiency by using various copper sources including CuSO2/ascorbic acid, CuSO4/TCEP, or CuBr. Data points represent the average of triplicate experiments. (B) Treatment with tunicamycin suppressed cell surface fluorescence (cells cultured with natural fucose in red, azidofucose in green, or azidofucose in the presence of tunicamycin in black). (C) Labeling with biotinylated alkyne 13 was performed with UltraAvidin-Fluorescein, then analyzed by flow cytometry (cells treated with fucose in red or azidofucose in green).
To demonstrate that azido-modified glycoproteins are displayed on the cell surface, we performed stepwise staining with a biotinylated alkyne reagent 13. The azidofucose-treated cells were labeled with biotinylated alkyne 13 and then stained with an avidin-fluorescein conjugate that is not taken up by the cells. There was a distinct increase of fluorescent signal when the cells were treated with azidofucose versus natural fucose (Fig. 5C), suggesting azido-modified fucose glycans are presented on the cell surface where they can be used as chemical handles for selective labeling. These results demonstrate that the azidofucose-treated cells express the azido-fucosylated glycoproteins on the cell surface. Moreover, they can be visualized selectively by using the alkyne probe via the Cu(I)-catalyzed [3 + 2] cycloaddition.
Visualization of Fucosylated Glycoconjugates Inside the Cell.
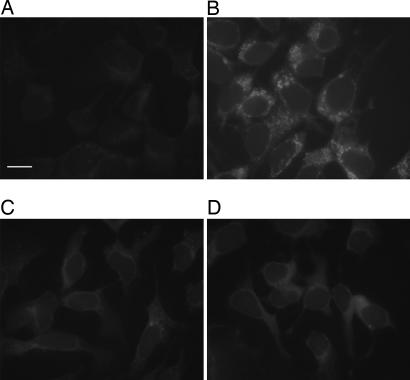
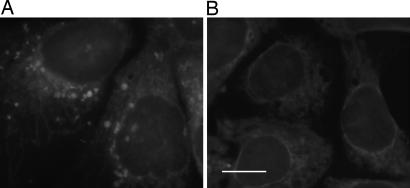
To demonstrate the feasibility of using the naphthalimide probe for intracellular imaging, we used fluorescence microscopy. The human hepatoma cell line, Hep 3B, was incubated with acetylated azidofucose 12. After 3 days, the cells were fixed, washed with PBS, and then stained with 1a for visualization. Compared with the cells incubated with natural fucose (Fig. 6A), a distinct punctate-labeling pattern was observed in cells incubated with azidofucose (Fig. 6B). To confirm that the imaging data were a click-activated phenomenon, cells were treated with a water-soluble trisulfonated triphenylphosphine to selectively reduce the azides before treatment with 1a. After reduction, the images showed minimal fluorescence, similar to the negative control (Fig. 7). This method provides sufficient a signal-to-noise ratio to allow direct analysis of azidofucosylation in whole cells. Next, double-staining experiments were performed to find out whether the punctate pattern observed from azidofucose marks specific cellular compartments. As shown in Fig. 8, azidofucose labeling overlapped perfectly with Alexa Fluor 594-conjugated wheat germ agglutinin (WGA) lectin staining. The WGA lectin binds to sialic acid and N-acetylglucosaminyl residues of glycoproteins and is commonly used as a Golgi marker. These data further document the specific nature and sensitivity of our fluorogenic probe, allowing visualization of fucosylated glycoproteins in whole cells.
Fig. 6.
Fluorescent image of cells labeled with probe 1a. The labeling reaction was performed in the presence of Cu(I) after the treatment with natural fucose (A) or azidofucose (B), or in the absence of Cu(I) after the treatment with natural fucose (C) or azidofucose (D). (Scale bar: 20 μm.)
Fig. 7.
Specificity of the Cu(I)-catalyzed cycloaddition for azidofucose. Azidofucose-supplemented cells were fixed and treated directly with probe (A) or were reduced with Tris(3-sulfonatophenyl)phosphine and then subjected to labeling reaction (B). (Scale bar: 20 μm.)
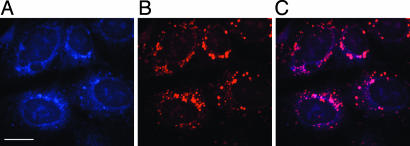
Fig. 8.
Imaging intracellular fucosylation by double staining with the probe 1a and WGA lectin. Azidofucose treated cells were fixed and labeled with probe 1a and then further treated with WGA lectin conjugated with Alexa Fluor 594, and the cells were imaged with confocal fluorescence microscope by using appropriate filter sets. (A) Blue fluorescence, labeled with probe 1a. (B) Red fluorescence, stained with Alexa Fluor 594-conjugated WGA lectin as a Golgi marker. (C) Purple color, overlap in blue and red signals. (Scale bar: 20 μm.)
Discussion
Altered fucosylation of glycoproteins has been observed in several pathological processes, including inflammation and tumorigenesis (44). Indeed, fucosylation of certain proteins serves as a marker of carcinoma progression and prognosis (2, 41, 45). For example, up-regulation of sialyl Lewisa and sialyl Lewisx has been documented in numerous cancers, and such increases correlate with metastatic potential and poor prognosis (46). Core-fucosylation of α-fetoprotein is also a marker for poor prognosis in hepatocellular carcinoma (47). In addition, glycosphingolipids are well known tumor-associated antigens (48). The cell surface glycosphingolipid Globo H is an epitope found on the cell surface of breast, prostate, and ovarian cancers (6, 49, 50). Work in this laboratory has demonstrated that the fucose residue in Globo H plays a critical role for anti-Globo H monoclonal antibody recognition (51).
Fucosylation events require the action of FucTs, which catalyze transfer from GDP-fucose to oligosaccharide chains conjugated to proteins and lipids. In general, fucose transfer is the final step in the synthesis of fucosylated glycoconjugates, such as selectin ligands that modulate cell adhesion and lymphocyte recirculation (52). To date, nine human FucTs have been isolated and characterized (53). In addition, two putative FucTs have been identified in the human genome (54, 55). FucTs are classified into three groups based on their sequence similarities and function: α1,2-, α1,3/4-, and α1,6 FucTs, attach a fucose residue in α2-, α3/4-, and α6 linkages to acceptor glycans, respectively (56). It has been reported that up-regulation of FucTs and their fucosylated products correlate with certain tumor progressions (47, 57). However, the mechanism of overexpression and the identity of FucTs related to the diseases still are unclear. Consequently, identification of specific fucosylated glycoconjugates associated with diseases correlated with differential activity of FucTs remains a high priority. Specific labeling of fucosylated glycans in the whole cell has great potential to help in the rapid profiling of such fucosylated glycoconjugates.
Described here is a click-activated fluorescent probe that is useful for labeling modified fucose glycans. This probe is especially practical in biological systems because a fluorescent signal is activated only after a highly selective, bioorthogonal ligation event. Using flow cytometry, we demonstrate that the nonfluorescent naphthalimide probe 1a selectively and fluorescently labels the fucosylated glycans on the cell surface after Cu(I)-catalyzed [3 + 2] cycloaddition with azide-bearing fucosides. The modified 6-azidofucose was fed to the cell in acetylated form facilitating incorporation into glycoproteins through the salvage biosynthetic pathway. A stepwise staining experiment by using biotinylated alkyne reagent 13 and an avidin-fluorescein conjugate confirmed that the cycloaddition reaction occurred on the cell surface. A significant reduction in the fluorescent signal was observed upon tunicamycin treatment, indicating that most azidofucose residues are presented in the context of N-linked glycoproteins. This result is consistent with the fact that cell surface glycans displayed on Jurkat cells are primarily N-linked glycoproteins, due to an O-glycosylation defect (58). Click-activated probe 1a also allowed for the imaging of fucosylated glycoconjugates in whole cells, which helped define their cellular localization. Significant fluorescent patterns were observed by fluorescence microscopy that closely matched the signals generated by a Golgi marker. The signal from glycoconjugates was extinguished when cells were treated with a water-soluble triphenylphosphine, indicating that the fluorogenic reaction proceeds selectively at azides present on fucose.
In conclusion, our click-activated fluorogenic-labeling technique permits imaging of fucosylated glycoconjugates at the cell surface and inside the cell. Because of the high structural complexity of carbohydrates and the diversity of glycans, many functions of fucosylated glycoconjugates remain to be elucidated. Fluorescent labeling represents a way to address some of the questions concerning the structure and function of fucosylated glycans. It facilitates a comparison of fucosylation in normal and tumor cells. It also allows monitoring of particular fucosylated glycoconjugates after inhibition of specific FucTs with small molecules or RNAi (39, 59, 60).
Materials and Methods
Analysis of Fluorescent Labeling at the Cell Surface by Flow Cytometry.
Jurkat cells were cultured in RPMI medium 1640 (Invitrogen, Carlsbad, CA), supplemented with 10% FCS and peracetylated fucose 11 or azidofucose 12 (200 μM) at a density of 2 × 105 cells per ml for 3 days, in the presence or absence of tunicamycin (5 μg/ml). After washing with 0.1% FCS/PBS, 106 cells were resuspended in 100 μl of a reaction solution (0.1 mM probe 1a or biotinylated alkyne 13/0.2 mM Tris-triazoleamine catalyst/0.1 mM CuBr in PBS) at room temperature for 30 min, followed by washing with 0.1% FCS/PBS. For the biotinylation experiment, cells were stained with 0.25 μg of UltraAvidin-Fluorescein (Leinco Technologies, St. Louis, MO) in 50 ml of staining buffer (1% FCS/0.1% NaN3 in PBS) for 30 min at 4°C, followed by three washes with staining buffer. The fluorescence intensity was detected and acquired by BD LSR II (BD Biosciences, San Jose, CA) and FACSDiva software (BD Biosciences). Twenty thousand events were collected in each sample. Data analysis was performed with CellQuest Pro software (BD Biosciences). For detection of the fluorescent adduct with probe 1a, a 351-nm UV laser was used for excitation, and emission was detected by a 440/40 band-pass filter.
Fluorescence Microscopy and Imaging.
The human hepatocellular carcinoma cell line, Hep3B (American Type Culture Collection, Manassas, VA), was cultured in Opti-MEM (Invitrogen) supplemented with 0.1% FCS and treated with natural fucose or peracetylated azidofucose 12 (200 μΜ) for 3 days. The cells then were transferred to a coverslip glass slide and cultured overnight in the same medium. The cells were fixed by acetone and labeled as follows. Fixed cells were incubated with 0.2 mM probe 1a/2.0 mM Tris-triazoleamine catalyst/1.0 mM CuSO4/2.0 mM sodium ascorbate in PBS at room temperature overnight. After labeling, cells were washed with PBS, and fluorescence images were obtained by using Axiovert 200M (Carl Zeiss, Inc., Thornwood, NY). For counter staining of Golgi compartments, the fixed cells were stained by using Alexa Fluor 594 conjugated WGA lectin (Invitrogen), and each fluorescent dye was imaged by using Bio-Rad (Carl Zeiss) Radiance 2100 Rainbow laser scanning confocal microscopy system.
Supporting Information.
Details describing the synthesis of the compounds and experimental procedures can be found in supporting information.
Supplementary Material
Acknowledgments
We thank Drs. William B. Kiosses and Shigeki Shimada for help with microscopy and Drs. Jinq-Chyi Lee and Douglass Wu for their helpful discussions. This research was supported by National Institutes for Health grants and The Skaggs Institute for Chemical Biology.
Glossary
Abbreviations
- AGP
α1-acid glycoprotein
- FucT
fucosyltransferase
- WGA
wheat germ agglutinin.
Footnotes
Conflict of interest statement: No conflicts declared.
References
- 1.Apweiler R., Hermjakob H., Sharon N. Biochim. Biophys. Acta. 1999;1473:4–8. doi: 10.1016/s0304-4165(99)00165-8. [DOI] [PubMed] [Google Scholar]
- 2.Becker D. J., Lowe J. B. Glycobiology. 2003;13:41R–53R. doi: 10.1093/glycob/cwg054. [DOI] [PubMed] [Google Scholar]
- 3.Staudacher E. Trends Glycosci. Glycotechnol. 1996;8:391–408. [Google Scholar]
- 4.Sears P., Wong C.-H. Cell. Mol. Life Sci. 1998;54:223–252. doi: 10.1007/s000180050146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Haltiwanger R. S., Lowe J. B. Annu. Rev. Biochem. 2004;73:491–537. doi: 10.1146/annurev.biochem.73.011303.074043. [DOI] [PubMed] [Google Scholar]
- 6.Dube D. H., Bertozzi C. R. Nat. Rev. Drug Discov. 2005;4:477–488. doi: 10.1038/nrd1751. [DOI] [PubMed] [Google Scholar]
- 7.Hirabayashi J. Glycoconj. J. 2004;21:35–40. doi: 10.1023/B:GLYC.0000043745.18988.a1. [DOI] [PubMed] [Google Scholar]
- 8.Shriver Z., Raguram S., Sasisekharan R. Nat. Rev. Drug Discov. 2004;3:863–873. doi: 10.1038/nrd1521. [DOI] [PubMed] [Google Scholar]
- 9.Khidekel N., Ficarro S. B., Peters E. C., Hsieh-Wilson L. C. Proc. Natl. Acad. Sci. USA. 2004;101:13132–13137. doi: 10.1073/pnas.0403471101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ratner D. M., Adams E. W., Disney M. D., Seeberger P. H. ChemBioChem. 2004;5:1375–1383. doi: 10.1002/cbic.200400106. [DOI] [PubMed] [Google Scholar]
- 11.Prescher J. A., Bertozzi C. R. Nat. Chem. Biol. 2005;1:13–21. doi: 10.1038/nchembio0605-13. [DOI] [PubMed] [Google Scholar]
- 12.Raman R., Raguram S., Venkataraman G., Paulson J. C., Sasisekharan R. Nat. Methods. 2005;2:817–824. doi: 10.1038/nmeth807. [DOI] [PubMed] [Google Scholar]
- 13.Chudakov D. M., Lukyanov S., Lukyanov K. A. Trends Biotechnol. 2005;23:605–613. doi: 10.1016/j.tibtech.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 14.Mahal L. K., Yarema K. J., Bertozzi C. R. Science. 1997;276:1125–1128. doi: 10.1126/science.276.5315.1125. [DOI] [PubMed] [Google Scholar]
- 15.Tai H. C., Khidekel N., Ficarro S. B., Peters E. C., Hsieh-Wilson L. C. J. Am. Chem. Soc. 2004;126:10500–10501. doi: 10.1021/ja047872b. [DOI] [PubMed] [Google Scholar]
- 16.Saxon E., Bertozzi C. R. Science. 2000;287:2007–2010. doi: 10.1126/science.287.5460.2007. [DOI] [PubMed] [Google Scholar]
- 17.Sampathkumar S.-G., Li A. V., Jones M. B., Sun Z., Yarema K. J. Nat. Chem. Biol. 2006;2:149–152. doi: 10.1038/nchembio770. [DOI] [PubMed] [Google Scholar]
- 18.Rostovtsev V. V., Green L. G., Fokin V. V., Sharpless K. B. Angew. Chem. Int. Ed. Engl. 2002;41:2596–2599. doi: 10.1002/1521-3773(20020715)41:14<2596::AID-ANIE2596>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 19.Kolb H. C., Sharpless K. B. Drug Discov. Today. 2003;8:1128–1137. doi: 10.1016/s1359-6446(03)02933-7. [DOI] [PubMed] [Google Scholar]
- 20.Zhou Z., Fahrni C. J. J. Am. Chem. Soc. 2004;126:8862–8863. doi: 10.1021/ja049684r. [DOI] [PubMed] [Google Scholar]
- 21.Sivakumar K., Xie F., Cash B. M., Long S., Barnhill H. N., Wang Q. Org. Lett. 2004;6:4603–4606. doi: 10.1021/ol047955x. [DOI] [PubMed] [Google Scholar]
- 22.de Silva A. P., Gunaratne H. Q. N., Gunnlaugsson T. Tetrahedron Lett. 1998;39:5077–5080. [Google Scholar]
- 23.McAdam C. J., Morgan J. L., Murray R. E., Robinson B. H., Simpson J. Aust. J. Chem. 2004;57:525–530. [Google Scholar]
- 24.Tonetti M., Sturla L., Bisso A., Zanardi D., Benatti U., De Flora A. Biochimie. 1998;80:923–931. doi: 10.1016/s0300-9084(00)88889-6. [DOI] [PubMed] [Google Scholar]
- 25.Zeitler R., Danneschewski S., Lindhorst T., Thiem J., Reutter W. J. Enzyme Inhib. 1997;11:265–273. doi: 10.3109/14756369709027655. [DOI] [PubMed] [Google Scholar]
- 26.Yurcheno P. D., Atkinson P. H. Biochemistry. 1975;14:3107–3114. doi: 10.1021/bi00685a011. [DOI] [PubMed] [Google Scholar]
- 27.Yurcheno P. D., Atkinson P. H. Biochemistry. 1977;14:944–953. doi: 10.1021/bi00624a021. [DOI] [PubMed] [Google Scholar]
- 28.Dube D. H., Bertozzi C. R. Curr. Opin. Chem. Biol. 2003;7:616–625. doi: 10.1016/j.cbpa.2003.08.006. [DOI] [PubMed] [Google Scholar]
- 29.Düffels A., Green L. G., Lenz R., Ley S. V., Vincent S. P., Wong C.-H. Bioorg. Med. Chem. 2000;8:2519–2525. doi: 10.1016/s0968-0896(00)00187-5. [DOI] [PubMed] [Google Scholar]
- 30.Srivastava G., Kaur K. J., Hindsgaul O., Palcic M. M. J. Biol. Chem. 1992;267:22356–22361. [PubMed] [Google Scholar]
- 31.Vogel C., Bergemann C., Ott A.-J., Lindhorst T. K., Thiem J., Dahlhoff W. V., Hällgren C., Palcic M. M., Hindsgaul O. Liebigs Ann. 1997:601–612. [Google Scholar]
- 32.Binch H., Stangier K., Thiem J. Carbohydr. Res. 1998;306:409–419. [Google Scholar]
- 33.Gilbert J. C., Weerasooriya U. J. Org. Chem. 1982;47:1837–1845. [Google Scholar]
- 34.Huisgen R. Angew. Chem. Int. Ed. Engl. 1963;2:565–632. [Google Scholar]
- 35.Chan T. R., Hilgraf R., Sharpless K. B., Fokin V. V. Org. Lett. 2004;6:2853–2855. doi: 10.1021/ol0493094. [DOI] [PubMed] [Google Scholar]
- 36.Lewis W. G., Magallon F. G., Fokin V. V., Finn M. G. J. Am. Chem. Soc. 2004;126:9152–9153. doi: 10.1021/ja048425z. [DOI] [PubMed] [Google Scholar]
- 37.Wittmann V., Wong C.-H. J. Org. Chem. 1997;62:2144–2147. doi: 10.1021/jo9620066. [DOI] [PubMed] [Google Scholar]
- 38.Fazio F., Bryan M. C., Blixt O., Paulson J. C., Wong C.-H. J. Am. Chem. Soc. 2002;124:14397–14402. doi: 10.1021/ja020887u. [DOI] [PubMed] [Google Scholar]
- 39.Bryan M. C., Lee L. V., Wong C.-H. Bioorg. Med. Chem. Lett. 2004;14:3185–3188. doi: 10.1016/j.bmcl.2004.04.001. [DOI] [PubMed] [Google Scholar]
- 40.Rydén I., Påhlsson P., Lindgren S. Clin. Chem. 2002;48:2195–2201. [PubMed] [Google Scholar]
- 41.Hashimoto S., Asao T., Takahashi J., Yagihashi Y., Nishimura T., Saniabadi A. R., Poland D. C., van Dijk W., Kuwano H., Kochibe N., Yazawa S. Cancer. 2004;101:2825–2836. doi: 10.1002/cncr.20713. [DOI] [PubMed] [Google Scholar]
- 42.Link A. J., Vink M. K. S., Tirrell D. A. J. Am. Chem. Soc. 2004;126:10598–10602. doi: 10.1021/ja047629c. [DOI] [PubMed] [Google Scholar]
- 43.Sarkar A. K., Fritz T. A., Taylor W. H., Esko J. D. Proc. Natl. Acad. Sci. USA. 1995;92:3323–3327. doi: 10.1073/pnas.92.8.3323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Walz G., Aruffo A., Kolanus W., Bevilacqua M., Seed B. Science. 1990;250:1132–1135. doi: 10.1126/science.1701275. [DOI] [PubMed] [Google Scholar]
- 45.Taniguchi N., Ekuni A., Ko J. H., Miyoshi E., Ikeda Y., Ihara Y., Nishikawa A., Honke K., Takahashi M. Proteomics. 2001;1:239–247. doi: 10.1002/1615-9861(200102)1:2<239::AID-PROT239>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 46.Kannagi R., Izawa M., Koike T., Miyazaki K., Kimura N. Cancer Sci. 2004;95:377–384. doi: 10.1111/j.1349-7006.2004.tb03219.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Miyoshi E., Noda K., Yamaguchi Y., Inoue S., Ikeda Y., Wang W., Ko J. H., Uozumi N., Li W., Taniguchi N. Biochim. Biophys. Acta. 1999;1473:9–20. doi: 10.1016/s0304-4165(99)00166-x. [DOI] [PubMed] [Google Scholar]
- 48.Hakomori S., Zhang Y. Chem. Biol. 1997;4:97–104. doi: 10.1016/s1074-5521(97)90253-2. [DOI] [PubMed] [Google Scholar]
- 49.Kannagi R., Levery S. B., Ishigami F., Hakomori S. I., Shevinsky L. H., Knowles B. B., Solter D. J. Biol. Chem. 1983;258:8934–8942. [PubMed] [Google Scholar]
- 50.Zhang S. L., Cordon-Cardo C., Zhang H. S., Reuter V. E., Adluri S., Hamilton W. B., Lloyd K. O., Livingston P. O. Int. J. Cancer. 1997;73:42–49. doi: 10.1002/(sici)1097-0215(19970926)73:1<42::aid-ijc8>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 51.Huang C.-Y., Thayer D. A., Chang A. Y., Best M. D., Hoffmann J., Head S., Wong C.-H. Proc. Natl. Acad. Sci. USA. 2006;103:15–20. doi: 10.1073/pnas.0509693102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Schottelius A. J., Hamann A., Asadullah K. Trends Immunol. 2003;24:101–104. doi: 10.1016/s1471-4906(03)00024-3. [DOI] [PubMed] [Google Scholar]
- 53.Javaud C., Dupuy F., Maftah A., Julien R., Petit J. M. Genetica. 2003;118:157–170. [PubMed] [Google Scholar]
- 54.Roos C., Kolmer M., Mattila P., Renkonen R. J. Biol. Chem. 2002;277:3168–3175. doi: 10.1074/jbc.M107927200. [DOI] [PubMed] [Google Scholar]
- 55.Baboval T., Smith F. I. Mamm. Genome. 2002;13:538–541. doi: 10.1007/s00335-001-2152-5. [DOI] [PubMed] [Google Scholar]
- 56.Oriol R., Mollicone R., Cailleau A., Balanzino L., Breton C. Glycobiology. 1999;9:323–334. doi: 10.1093/glycob/9.4.323. [DOI] [PubMed] [Google Scholar]
- 57.Staudacher E., Altmann F., Wilson I. B. H., März L. Biochim. Biophys. Acta. 1999;1473:216–236. doi: 10.1016/s0304-4165(99)00181-6. [DOI] [PubMed] [Google Scholar]
- 58.Piller V., Piller F., Fukuda M. J. Biol. Chem. 1990;265:9264–9271. [PubMed] [Google Scholar]
- 59.Mitchell M. L., Tian F., Lee L. V., Wong C.-H. Angew. Chem. Int. Ed. Engl. 2002;41:3041–3044. doi: 10.1002/1521-3773(20020816)41:16<3041::AID-ANIE3041>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 60.Lee L. V., Mitchell M. L., Huang S.-J., Fokin V. V., Sharpless K. B., Wong C.-H. J. Am. Chem. Soc. 2003;125:9588–9589. doi: 10.1021/ja0302836. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.