Abstract
Since the discovery of calbindin D9k, its role in intestinal calcium absorption has remained unsettled. Further, a wide distribution of calbindin D9k among tissues has argued for its biological importance. We discovered a frameshift deletion in the calbindin D9k gene in an ES cell line, E14.1, that originated from 129/OlaHsd mice. We produced mice with the mutant calbindin D9k gene by injecting the E14.1 ES cell subline into the C57BL/6 host blastocysts and proved that these mice lack calbindin D9k protein. Calbindin D9k knockout mice were indistinguishable from wild-type mice in phenotype, were able to reproduce, and had normal serum calcium levels. Thus, calbindin D9k is not required for viability, reproduction, or calcium homeostasis.
Keywords: vitamin D3, E14ES cells, calcium homeostasis
For decades, a major physiologic function of calbindin D9k was believed to be a carrier of calcium during intestinal calcium absorption (1). However, the detailed mechanism of vitamin D-induced intestinal calcium absorption still is not fully understood. According to the currently accepted model, vitamin D-mediated transcellular calcium absorption in the intestine proceeds through the calcium channel proteins TRPV5 and TRPV6 (2) with the involvement of cellular calcium transfer protein calbindin D9k (1, 3) and a calcium extrusion protein, calcium ATPase (PMCA1b) (4). The data on regulation of epithelial calcium channels (TRPV5 and TRPV6), calbindin D9k, and PMCA1b expression by 1,25-dihydroxyvitamin D3 seem clear, and these genes have vitamin D responsive elements in their promoter regions (5–7).
Calbindin D9k was first discovered as the mammalian counterpart to the calbindin D28k that was found in chick duodenal mucosa in response to vitamin D3 (8, 9). Later, calbindin D9k from vitamin D3-responsive rat intestinal mucosa was identified, purified, and characterized (9, 10). Since its discovery in 1967, the role of calbindin D9k protein in vitamin D3-mediated intestinal calcium absorption has been intensively studied but still remains unsettled. In the meantime, a wide distribution of this protein in tissues not involved in calcium absorption has been noted, arguing for its importance in biology (11–13).
In 1969, Harmeyer and DeLuca (14) provided evidence that calcium absorption and calbindin D9k levels in response to vitamin D3 are not directly related. Similar discrepancies were reported by others (15). Additional doubts concerning the role of calbindin D9k in calcium transport appeared after studies in vitamin D receptor knockout mice (16, 17). The absence of a calbindin D9k-mediated mechanism for active Ca2+ transport was also shown in sheep rumen (18). In our microarray experiments, we demonstrated that expression of the calbindin D9k gene was only slightly up-regulated by 1,25-dihydroxyvitamin D3 [1,25-(OH)2D3] in the intestine of vitamin D3-deficient rats (19). We also found that, although administration of some analogs of 1,25-(OH)2D3 stimulated Ca2+ absorption in the intestine of vitamin D-deficient rats, the expression of calbindin D9k was not changed.
We have now produced a strain of mice lacking the calbindin D9k protein. This mouse is indistinguishable from the wild type in phenotype and in serum calcium level, regardless of age or gender. Additionally, these mice are able to reproduce. Thus, calbindin D9k is not required for viability, reproduction, or calcium homeostasis.
Results and Discussion
Discovery of the Mutant Mouse Calbindin D9k Gene.
The P1 clone 7681 with an 85-kb insert of genomic DNA/SauIIIA containing the mouse calbindin D9k gene was purchased from Incyte Genomics (Palo Alto, CA). The P1 mouse library was generated by Sternberg et al. (20) by using genomic DNA from the ES cell line subclone E14.1, which is derived from E14 ES cells (21). The ES cell line E14 was derived from the inbred mouse strain 129/OlaHsd in 1985 by Hooper et al. (22). The insert was cut with BamHI and subcloned into pBluescript (Stratagene, La Jolla, CA). The clone that was positive for the calbindin D9k gene was selected by Northern blot analysis, and a plasmid with the mutant calbindin D9k insert was isolated. We have sequenced the 10.409-kb insert containing the calbindin D9k gene and compared it with the 8.434 kb of deposited sequence of the calbindin D9k gene of mouse strain ICRxSwiss (GenBank accession no. AY034822). Both sequences overlap in the calbindin D9k gene region, but our sequence is 2,111 bp downstream of the AY034822 sequence. Both sequences seemed to be 98–99% identical, with a few gaps in noncoding regions. The major difference we discovered in the calbindin D9k gene sequence was the deletion of 22 nt in exon III compared with AY034822 (Fig. 1). This deletion resulted in a frameshift, thereby creating a new in-frame stop codon 63 nt downstream of the original stop codon (Fig. 1), which could generate a hypothetical mutant calbindin D9k protein that would be 21 aa longer than the native protein.
Fig. 1.
Structure of the mutant calbindin D9k gene from ES cell line E14.1. Exons are underlined; signaling sequences are highlighted.
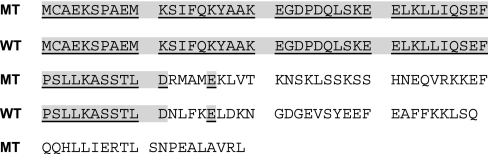
The deduced mutant protein should be 100 aa long, with a molecular weight of 11,363 and a theoretical pI of 9.07 with a total of 14 negatively charged residues (Asp plus Glu) and a total of 17 positively charged residues (Arg plus Lys). The wild-type protein is 79 aa, with a molecular weight of 8,970 and a theoretical pI of 4.69 with a total of 16 negatively charged residues (Asp plus Glu) and a total of 11 positively charged residues (Arg plus Lys). We aligned sequences of the wild-type protein and the deduced mutant calbindin D9k protein (Fig. 2). Both proteins are identical in the first 51 aa and have no similarity after that. Wild-type calbindin D9k protein, as a Ca2+-binding protein, has two EF hands and is acidic (23), whereas the mutant calbindin D9k is missing the second EF hand and is basic. We assumed that this protein might not exist in vivo, as was shown for caspase 3 (24), so mice with this mutant calbindin D9k gene could potentially be calbindin D9k null.
Fig. 2.
Sequence alignment for mutant and wild-type calbindin D9k proteins.
Generation of Mutant Calbindin D9k Gene Mice.
We first checked whether the original 129/OlaHsd mice, which were the source of the ES cell line E14.1, have the same 22-nt deletion in the calbindin D9k gene, but they did not. We checked several other 129 mouse substrains, and all of them had the wild-type calbindin D9k genotype. So the only source of the mutant calbindin D9k gene was the ES cell subclone E14.1.
We generated mice with the mutant calbindin D9k gene by injection of the E14.1 ES cell subline into C57BL/6 host blastocysts as described in Materials and Methods. Chimeric males were bred to 129/OlaHsd females to generate an inbred 129/OlaHsd line that was homozygous for the mutant calbindin D9k gene with a 22-nt deletion in exon III as was confirmed by genotyping.
Mutant Calbindin D9k Mice Have the Knockout Phenotype.
Mice homozygous for the mutant calbindin D9k gene (22 nt deleted in exon III) were generated and mRNA analyzed by RT-PCR. For this, we used primers flanking the entire coding region of the calbindin D9k gene. These primers (see Materials and Methods) amplified a 418-bp product from wild-type mice and a 396-bp product from the mutant mice. As expected, no full-length product (418 bp) was detected in mutant calbindin D9k mice. Both products were sequenced to confirm the specificity of the amplification. Sequencing data revealed that the PCR product obtained from the mRNA of mutant mice had the identical 22-bp deletion detected in exon III of the mutant calbindin D9k gene.
To determine whether either the mutant or wild-type calbindin D9k protein could be detected in the mutant mouse duodenum lysate, we used all available antibodies. Western blot analysis was carried out by using duodenal lysates from the wild-type and mutant mice. As shown in Fig. 3A, an antibody raised against the full-length calbindin D9k protein (1–79 aa) recognized a 9-kDa band in the wild type; however, neither a 9-kDa band (wild-type protein) nor an 11.3-kDa (mutant protein) band was detected in the mutant mice duodenal lysates. The antibody to the common segment (1–60 aa) of both wild-type and putative mutant calbindin D9k proteins detected the wild-type protein band but failed to detect either of these bands in the mutant duodenal lysate (Fig. 3B).
Fig. 3.
Western blots of calbindin D9k from wild-type and mutant duodenum lysates. (A) Results obtained by using antibody to full-length (1–79 aa) calbindin D9k protein. (B) Results obtained by using antibody to partial (1–60 aa) calbindin D9k protein. (C) Results obtained by using antibody developed to mutant calbindin D9k protein (1–100 aa) and using purified recombinant mutant calbindin D9k (MUT P) as the control.
To be sure that the mutant calbindin D9k protein is not expressed in mutant mice, we expressed the mutant protein in Escherichia coli, purified it to homogeneity, and produced antibodies to the mutant calbindin D9k protein. Using this antibody, no mutant calbindin D9k protein was found in mutant mice duodenal lysates, whereas this antibody clearly showed calbindin D9k in the lysates of wild-type mice (Fig. 3C).
These results clearly demonstrate that putative mutant calbindin D9k protein does not exist in the mutant mouse despite the presence of its mRNA, thus confirming that the mouse that is homozygous for the mutant calbindin D9k gene is a calbindin D9k null mouse.
Calbindin D9k Knockout Mice Phenotype and Serum Calcium Level.
The calbindin D9k knockout mice do not exhibit any overt phenotypic abnormalities: specifically, no skeletal abnormalities were detected. They grow and develop normally; the growth rate of the weanlings is comparable to that of the wild type as assessed by their weekly weight gain. Their life expectancy also does not differ from the wild type. Calbindin D9k knockout mice retain similar fertility to the wild-type mice and are fully able to reproduce. The average size of the litter from the knockout is comparable to that of wild type as well. To find out whether the lack of calbindin D9k protein in the knockout mice results in any effect on calcium homeostasis, serum calcium levels of the knockout and wild-type mice kept on a chow diet were monitored from 4 weeks of age to adulthood (Fig. 4). The data show that the serum calcium levels of the knockout and the wild-type mice are identical over the extended period investigated in both male and female mice.
Fig. 4.
Concentration of Ca2+ in the serum of wild-type and calbindin D9k knockout mice as a function of age and sex. Serum calcium levels in the wild-type and knockout mice were measured every 4 weeks (see Materials and Methods). (A) Serum calcium levels in female mice. (B) Serum calcium levels in male mice.
Thus, we generated mice lacking the calbindin D9k protein and demonstrated that knockout mice are indistinguishable from wild type in phenotype and are able to maintain normal serum calcium levels in the absence of calbindin D9k protein, regardless of age or sex. Although a direct assessment of whether the intestine lacking the calbindin D9k protein can respond to 1,25-dihydroxyvitamin D3 by increasing calcium absorption has not yet been examined, the fact that serum calcium is normal in knockout mice suggests that calbindin D9k is not required for vitamin D-induced calcium absorption. This belief is supported by the fact that vitamin D receptor null mice are hypocalcemic under these circumstances (25).
Materials and Methods
Calbindin D9k Gene Insert.
P1 clone 7681 with an 85-kb genomic DNA/SauIIIA insert containing the calbindin D9k gene from the mouse E14.1 ES cell P1-ES Library was purchased from Incyte Genomics.
ES Cell Lines.
The ES cell line E14 was derived from the inbred mouse strain 129/OlaHsd in 1985 by Hooper et al. (22). Later, it was subcloned by Kuhn et al. (21) to become the E14.1 line. The E14.1B and E14.1C ES cell lines were obtained from Philip Sanford (Gene Targeted Mouse Service, University of Cincinnati, Cincinnati, OH).
Mice.
129/OlaHsd mice were purchased from Harlan (Indianapolis, IN). Animals were maintained and research was conducted in accordance with guidelines set forth by the Animal Care and Research Committee of University of Wisconsin.
Identity of the 129/Ola Calbindin D9k Gene.
The sequence was established by sequencing the DNA on an LKB ALF DNA sequencer (GE Healthcare Bio-Sciences, Piscataway, NJ) by using the Thermo sequenase fluorescent-labeled primer-cycle sequencing kit (GE Healthcare Bio-Sciences) at the University of Wisconsin Biotechnology Center DNA Synthesis/Sequencing Facility, using the set of 20 primers designed for each direction. Computer analysis of the sequence was performed with the Sequence Analysis Software Package from Genetics Computer Group (Madison, WI).
Generation of Mutant Calbindin D9k Mice.
The E14.11C ES cell subline was injected into C57BL/6 host blastocysts at the University of Wisconsin Biotechnology Center Transgenic Animal Facility. ES cells were grown for 3–4 days on a leukemia inhibitory factor-producing feeder layer. ES cells were disaggregated into a single-cell suspension, separated from the feeder cells, and injected into the blastocoel cavities of expanded C57BL/6 blastocysts. After the microinjections, the blastocysts were allowed to recover and transferred into the oviducts of pseudopregnant recipients. Potential founder pups were born 19 days later, and coat color was identified 1 week later. The 129/OlaHsd ES cells were from an agouti strain, and the blastocysts were from a black strain. Nine males with a high percentage of agouti hair were produced.
Three founder chimeras were mated to C57BL/6 partners at 6–7 weeks of age. All three founders produced agouti pups, indicating that the injected ES cells contributed to the formation of the germ line in the founder animals. Agouti F1 pups were genotyped by PCR.
Chimeric males were bred to 129/OlaHsd females (Harlan) to generate an inbred 129/Ola line. All female F1 pups were heterozygous for the calbindin D9k deletion. The heterozygous pups were backcrossed to founder males to generate female mice that were homozygous for the calbindin D9k gene with deletion.
Genotyping of Calbindin D9k Knockout Mice.
Genomic DNA was extracted from mouse tails by using a Puregene DNA isolation kit (mouse tail kit, Gentra Systems, Minneapolis, MN). To distinguish the wild-type allele from mutant alleles, a separate set of primers was designed for each allele. For the wild-type allele, the downstream primer was designed within the 22-bp deletion region of exon III of the calbindin gene. Because the mutant allele lacks this 22-bp region, this primer is unable to bind to the mutant allele. On the other hand, the downstream primer for the mutant allele was designed for only the flanking region of the deletion. The sequence of the primer covers part of the left border and part of the right border of the deletion. Therefore, the mutant downstream primer was unable to bind to the wild-type allele for PCR. The upstream primers were also designed for different regions of the gene upstream. The oligonucleotide primers designed for the wild-type allele were as follows: upstream, 5′-GAT CAT AGT GGG TTT CAG G-3′; downstream, 5′-ATC GCC ATT CTT ATC CAG-3′. The oligonucleotide primers designed for the mutant allele were as follows: upstream, 5′-CAC CCC ACC GAC CAT CAG-3′; downstream, 5′-ATC GCC ATT CTG TCC AGA GT-3′. The size of the wild-type PCR product is 324 bp, and the mutant PCR product is 189 bp.
Two separate PCRs were run for genomic DNA extracted from each mouse. One reaction was run with primer sets designed for wild-type alleles and another reaction was run with primer sets designed for mutant alleles. PCRs were performed by using the Advantage cDNA Polymerase Mix from Clontech Laboratories (Mountain View, CA). The parameters for PCR were as follows: initial denaturation at 94°C for 4 min followed by 29 cycles at 94°C for 30 s, 64°C for 40 s, and 72°C for 2 min. Final extension of the DNA was done for 5 min at 72°C. PCR was performed by using the Programmable Thermal Cycler PTC 100 (MJ Research, now Global Medical Instrumentation, Ramsey, MN). The PCR products were visualized on a 1.3% agarose gel run in 1XTAE after staining with ethidium bromide.
Intestinal Homogenate Preparation for mRNA Isolation.
The first 5 cm of intestine (the duodenum) was removed, slit open longitudinally, and scraped with a glass slide to remove mucosa. The mucosa was homogenized with a PowerGen 700 (Fisher Scientific, Pittsburgh, PA) in guanidine thiocyanate extraction buffer supplemented with 2% 2-mercaptoethanol (PolyATtract System 1000, Promega, Madison, WI), flash-frozen in liquid N2, and stored at −80°C.
mRNA Isolation.
Poly(A+) RNA was isolated from homogenized mucosa by using a PolyATtract System 1000 (Promega) and purified by using an RNeasy kit (Qiagen, Chatsworth, CA). The quality, integrity, and quantity of the poly(A+) RNA were determined by agarose gel electrophoresis and UV absorption spectrophotometry.
Reverse Transcription and DNA Amplification by PCR.
Reverse transcription of intestinal poly(A)+ RNA and subsequent DNA amplification was carried out by using a GeneAmp Gold RNA PCR reagent kit from Applied Biosystems (Foster City, CA) according to the conditions recommended by the manufacturer. The oligonucleotide primers used for DNA amplification were as follows: sense (upstream), 5′-CCT GCT GTT CCT GTC TGA-3′; antisense (downstream), 5′-CGT GTC TCC GAA CTT GCT TTA T-3′. The primers were designed to flank the entire coding region of the mRNA. The RT-PCR was performed on a Programmable Thermal Cycler PTC 100 (Global Medical Instrumentation). After amplification by PCR, the DNA reaction products were analyzed on 1.4% agarose gel after staining with ethidium bromide. The sequencing of the PCR product was carried out with an LKB ALF DNA sequencer (GE Healthcare Bio-Sciences) by using the Thermo sequenase fluorescent-labeled primer-cycle sequencing kit (GE Healthcare Bio-Sciences). Computer analysis of the sequence was performed by using the Sequence Analysis Software Package from Genetics Computer Group.
Construction of Plasmid with Mutant Calbindin D9k cDNA Sequence.
In the mutant calbindin D9k cDNA produced by RT-PCR as described above, restriction sites NdeI and BamHI were incorporated into the 5′ and 3′ sites, respectively. The NdeI site was located upstream of mutant calbindin D9k cDNA, and the BamHI site was located right after the stop codon. Using the generated restriction sites, mutant calbindin D9k cDNA was subcloned into the expression vector pET-14b (Novagen/EMD Biosciences, Madison, WI). This construct was transfected into XL-1 Blue Cells (Stratagene). The plasmid with the mutant calbindin D9k cDNA, p14D9Kmt, was isolated, and the sequence of mutant calbindin D9k cDNA was confirmed. In this construct, the mutant calbindin D9k was expressed as protein with the N-terminal His-tag sequence along with a thrombin cleavage site that was present between the His-tag and start of mutant calbindin D9k protein sequence.
Expression of Mutant Calbindin D9k Protein and E. coli Lysate Preparation.
The p14D9Kmt was transfected into BL21-CodonPlus (DE3)-RIPL Competent Cells (Stratagene) to express the mutant calbindin D9k according to manufacturer’s protocol. Cells were grown at 37°C, and the expression of the protein was induced at room temperature by 50 μΜ isopropyl β-d-thiogalactoside. E. coli cells were harvested 6 h later and lysed as follows. Cells (7.7 g) were added to 25 ml of lysis buffer containing 50 mM Tris·HCl (pH 8), 0.5 M NaCl, 20 mM imidazole, 0.02% sodium azide, 10 mM 2-mercaptoethanol, 20% glycerol, 1 mM PMSF, 1% Triton X-100, and one Complete, EDTA-Free Protease Inhibitor Mixture Tablet (Roche Diagnostics, Mannheim, Germany). The 2-mercaptoethanol, PMSF, Complete, EDTA-Free Protease Inhibitor Mixture Tablet, and Triton X-100 were added to the buffer immediately before adding the cells. Cells were lysed by sonication while keeping the lysis mixture cool with an ice/salt bath. The lysate was cleared of cellular debris by centrifugation at 12,851 × g at 4°C for 45 min and then analyzed on 16.5% Tris-Tricine gel.
Purification of Mutant Calbindin D9k.
The lysate was poured into a 50-ml conical tube containing 3 ml of NiNTA Superflow (Qiagen) as a 50% slurry in lysis buffer (see above). The mixture was rocked for 1 h at 4°C. The slurry was packed into a 1.5-cm-diameter column, and flow-through was collected. The column was washed with 10 bed volumes of lysis buffer. The bound protein was eluted with lysis buffer containing 150 mM imidazole. Protein levels in the effluent were monitored. A 20-ml volume was collected at the peak protein concentration and dialyzed two times against 2 liters of 20 mM Tris·HCl, pH 8.0/1 mM EDTA/1 mM DTT/20% glycerol/0.02% sodium azide. After dialysis, the solution was filtered through a 0.45-μm filter and loaded onto a 2.5 × 5.7-cm column of Q-Sepharose (GE Healthcare Bio-Sciences) equilibrated with the dialysis buffer. We found that the mutant calbindin D9k does not bind to Q-Sepharose and appears as a single protein in a flow-through (verified by SDS/PAGE), whereas contaminating proteins do bind to Q-Sepharose and could be eluted with a gradient buffer (0–500 mM NaCl/20 mM Tris·HCl, pH 8.0/1 mM EDTA). After washing the Q-Sepharose column with dialysis buffer until all nonbound mutant calbindin D9k was eluted, these fractions were collected and analyzed on 15% Tris-glycine gel. After SDS/PAGE, the pooled fractions containing pure mutant calbindin D9k (40 ml total) were brought to a final concentration of 25 mM Ca2+ by the addition of 2.0 M CaCl2, transferred to a 6.4 ml/cm 3,500 molecular weight cutoff Spectra/POR molecular porous membrane tube (Spectrum Medical Industries, Los Angeles, CA), and concentrated against PEG 2000 flakes. After 7 h, the sample was dialyzed overnight in 2 liters of buffer containing 25 mM Tris·HCl (pH 8.0), 25 mM CaCl2, 0.02% sodium azide, and 20% glycerol. The protein was concentrated further by using PEG 2000 flakes and then dialyzed overnight against a buffer containing 25 mM Tris·HCl (pH 8.0), 25 mM CaCl2, 20% glycerol, and 0.02% sodium azide. After dialysis, the total protein concentration was determined by the Bradford assay (26).
Removal of His-Tag from the Purified Mutant Calbindin D9k.
His-tag from the N-terminal site of the mutant calbindin D9k was removed by using a Thrombin Cleavage Capture kit from Novagen/EMD Biosciences). Removal of the His-tag was verified by SDS/PAGE by using 16.5% Tris-Tricine gel. After thrombin cleavage, only three extra amino acids (GSH) were left at the N terminus of the mutant calbindin D9k protein, resulting in a molecular mass of 11.644 kDa.
Production of Antibody to Mutant Calbindin D9k.
Polyclonal antibodies to the mutant calbindin D9k were derived from rabbits and affinity-purified by Sigma (St. Louis, MO). The antibodies were tested for their ability to detect the mutant protein by Western blot analysis.
Intestinal Lysate Preparation for Western Blot.
The first 5 cm of the duodenum was excised from the mouse immediately after carbon dioxide asphyxiation and immediately washed thoroughly with ice-cold saline. The duodenum was then homogenized by using a PowerGen 700 (Fisher Scientific) for 1 min in 1× PBS (pH 7.4) supplemented with 5 mM PMSF and Complete Mini Protease Inhibitor Mixture Tablets (one tablet per 7 ml of PBS) from Roche Diagnostics. One milliliter of PBS was used per 0.28 g of tissue. The homogenized samples were centrifuged twice for 15 min at 17,382 × g. All steps were performed on ice or at 4°C. The total protein concentration of the lysate (supernatant) was determined by the method of Bradford (26), using BSA as a standard. The lysates were aliquoted and stored at −80°C until analysis.
Western Blots.
Thirty micrograms of total protein from the wild-type and mutant mouse duodenum lysates were run on 16.5% Tris-Tricine gel (BioRad Laboratories, Hercules, CA), and the protein bands were transferred to an Immobilon PSQ membrane (Millipore, Bedford, MA) by using the TransBlot SD Semidry System (BioRad Laboratories). The transfer was carried out at 15 V for 31 min. Blots were blocked with 5% nonfat dry milk solution for 1 h at room temperature. After blocking, the blots were incubated with a rabbit anti-human calbindin D9k polyclonal antibody (H-60, sc-28532, 1:100, Santa Cruz Biotechnology, Santa Cruz, CA), a rabbit anti-rat recombinant calbindin D9k polyclonal antibody (1:1,000, SWANT, Bellinzona, Switzerland), or a custom-made rabbit anti-mouse mutant calbindin D9k polyclonal antibody (1:1,000). Finally, blots were incubated with peroxidase-labeled goat anti-rabbit IgG (1:500,000) from KPL (Gaithersburg, MD). Immunoreactive protein was detected with the Immobilon Western Chemiluminescence HRP substrate from Millipore.
Acknowledgments
We thank Patricia A. Powers and Joe Warren (both of the University of Wisconsin Biotechnology Center Transgenic Animal Facility) for generating the mutant calbindin D9k mice, Xiaohong Ma and Wendy Hellwig (both of the University of Wisconsin) for analytical work and animal care, Eric Danielson and Colleen Jones for assistance, and Pat Mings for help with the manuscript preparation. This work was supported by the Wisconsin Alumni Research Foundation and Comprehensive Cancer Center Grant P30 CA14520.
Footnotes
Conflict of interest statement: No conflicts declared.
References
- 1.Jones G., Strugnell S. A., DeLuca H. F. Physiol. Rev. 1998;78:1193–1231. doi: 10.1152/physrev.1998.78.4.1193. [DOI] [PubMed] [Google Scholar]
- 2.Hoenderop J. G. J., van Leeuwen J. P. T. M., van er Eerden B. C. J., Kersten F. F. J., van derKemp A. W. C. M., Mérillat A.-M., Waarsing J. H., Rossier B. C., Vallon V., Hummler E., Bindels R. J. M. J. Clin. Invest. 2003;112:1906–1914. doi: 10.1172/JCI19826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Christakos S., Barletta F., Huening M., Dhawan P., Liu Y., Porta A., Peng X. J. Cell. Biochem. 2003;88:238–244. doi: 10.1002/jcb.10349. [DOI] [PubMed] [Google Scholar]
- 4.Bouillon R., Van Cromphaut S., Carmeliet G. J. Cell. Biochem. 2003;88:332–339. doi: 10.1002/jcb.10360. [DOI] [PubMed] [Google Scholar]
- 5.Darwish H. M., DeLuca H. F. Arch. Biochem. Biophys. 1996;334:223–234. doi: 10.1006/abbi.1996.0450. [DOI] [PubMed] [Google Scholar]
- 6.Glendenning P., Ratajczak T., Prince R. L., Garamszegi N., Strehler E. E. Biochem. Biophys. Res. Commun. 2000;277:722–728. doi: 10.1006/bbrc.2000.3745. [DOI] [PubMed] [Google Scholar]
- 7.Colnot S., Ovejero C., Romagnolo B., Porteu A., Lacourte P., Thomasset M., Perret C. Endocrinology. 2000;141:2301–2308. doi: 10.1210/endo.141.7.7557. [DOI] [PubMed] [Google Scholar]
- 8.Wasserman R. H., Taylor A. N. Science. 1966;152:791–793. doi: 10.1126/science.152.3723.791. [DOI] [PubMed] [Google Scholar]
- 9.Kallfelz F. A., Taylor A. N., Wasserman R. H. Proc. Soc. Exp. Biol. Med.; 1967. pp. 54–58. [DOI] [PubMed] [Google Scholar]
- 10.Drescher D., DeLuca H. F. Biochemistry. 1971;10:2302–2307. doi: 10.1021/bi00788a019. [DOI] [PubMed] [Google Scholar]
- 11.Thomasset M. In: Vitamin D. 1st Ed. Feldman D., Glorieux F. H., Pike J. W., editors. San Diego: Academic; 1997. pp. 223–232. [Google Scholar]
- 12.Tebben P., Kumar R. In: Vitamin D. 2nd Ed. Feldman D., Pike J. W., Glorieux F. H. W., editors. San Diego: Elsevier Academic; 2005. pp. 515–536. [Google Scholar]
- 13.Christakos S., Liu Y., Dhawan P., Peng X. In: Vitamin D. 2nd Ed. Feldman D., Pike J. W., Glorieux F. H. W., editors. San Diego: Elsevier Academic; 2005. pp. 721–735. [Google Scholar]
- 14.Harmeyer J., DeLuca H. F. Arch. Biochem. Biophys. 1969;133:247–254. doi: 10.1016/0003-9861(69)90452-4. [DOI] [PubMed] [Google Scholar]
- 15.Spencer P., Charman M., Wilson P., Lawson E. Nature. 1976;263:161–163. doi: 10.1038/263161a0. [DOI] [PubMed] [Google Scholar]
- 16.Song Y., Kato S., Fleet J. C. J. Nutr. 2003;133:374–380. doi: 10.1093/jn/133.2.374. [DOI] [PubMed] [Google Scholar]
- 17.Krisinger J., Strom M., Darwish H. D., Perlman K., Smith C., DeLuca H. F. J. Biol. Chem. 1991;266:1910–1913. [PubMed] [Google Scholar]
- 18.Schroder B., Goebel W., Huber K., Breves G. J. Vet. Med. A Physiol. Pathol. Clin. Med. 2001;48:353–363. doi: 10.1046/j.1439-0442.2001.00369.x. [DOI] [PubMed] [Google Scholar]
- 19.Kutuzova G. D., DeLuca H. F. Arch. Biochem. Biophys. 2004;432:152–166. doi: 10.1016/j.abb.2004.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sternberg N., Smoller D., Braden T. Genet. Anal. Tech. Appl. 1994;11:171–180. doi: 10.1016/1050-3862(94)90038-8. [DOI] [PubMed] [Google Scholar]
- 21.Kuhn R., Rajewsky K., Muller W. Science. 1991;254:707–710. doi: 10.1126/science.1948049. [DOI] [PubMed] [Google Scholar]
- 22.Hooper M., Hardy K., Handyside A., Hunter S., Monk M. Nature. 1987;326:292–295. doi: 10.1038/326292a0. [DOI] [PubMed] [Google Scholar]
- 23.Kordel J., Skelton N. J., Akke M., Chazin W. J. J. Mol. Biol. 1993;231:711–734. doi: 10.1006/jmbi.1993.1322. [DOI] [PubMed] [Google Scholar]
- 24.Janicke R. U., Sprengart M. L., Wati M. R., Porter A. G. J. Biol. Chem. 1998;273:9357–9360. doi: 10.1074/jbc.273.16.9357. [DOI] [PubMed] [Google Scholar]
- 25.Yoshizawa T., Handa Y., Uematsu Y., Takeda S., Sekine K., Yoshihara Y., Kawakami T., Alioka K., Sato H., Uchiyama Y., et al. Nat. Genet. 1997;16:391–396. doi: 10.1038/ng0897-391. [DOI] [PubMed] [Google Scholar]
- 26.Bradford M. M. Anal. Biochem. 1965;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]