Abstract
Flaviviruses, such as West Nile virus (WNV), are significant human pathogens. The humoral immune response plays an important role in the control of flavivirus infection and disease. The structure of WNV complexed with the Fab fragment of the strongly neutralizing mAb E16 was determined to 14.5-Å resolution with cryo-electron microscopy. E16, an antibody with therapeutic potential, binds to domain III of the WNV envelope glycoprotein. Because of steric hindrance, Fab E16 binds to only 120 of the 180 possible binding sites on the viral surface. Fitting of the previously determined x-ray structure of the Fab–domain III complex into the cryo-electron microscopy density required a change of the elbow angle between the variable and constant domains of the Fab. The structure suggests that the E16 antibody neutralizes WNV by blocking the initial rearrangement of the E glycoprotein before fusion with a cellular membrane.
Keywords: cryo-electron microscopy, flavivirus, neutralization
West Nile virus (WNV) causes a febrile illness in humans that can progress to encephalitis, paralysis, and death. Although endemic in parts of Africa, Asia, Europe, and the Middle East, it was first isolated in the United States in 1999 and has subsequently spread throughout North America and the Caribbean (1–3). The similar Kunjin virus is found in Australia (4). WNV, a member of the Flaviviridae family, is closely related to other arthropod-borne, medically important viruses, such as dengue, yellow fever, Japanese encephalitis, and tick-borne encephalitis viruses. These small, ≈500-Å-diameter, lipid-enveloped viruses enter their host cells by receptor-mediated endocytosis. A low pH-triggered conformational rearrangement of the viral surface glycoproteins resulting in a fusion-active state of the virion allows the release of the single-stranded, positive-sense RNA genome from the endosomes into the cytoplasm.
The outer surface of mature infectious particles is formed by an icosahedral scaffold of 90 homodimers of the membrane-anchored envelope glycoprotein (E). The three E monomers per icosahedral asymmetric unit each have distinctly different environments (5, 6), contrary to most other icosahedral viruses in which all subunits have at least quasi-similar environments. The ectodomain of E consists of three structural domains, DI, DII, and DIII (7–9). DI is structurally positioned between DII and DIII. The dimerization DII contains a 12-residue-long loop essential for virus–cell membrane fusion and, in dengue virus, has carbohydrate moieties that mediate receptor binding (10). The C-terminal DIII undergoes a major, pH-triggered, positional rearrangement essential for fusion and may also be involved in receptor binding (7, 11–18).
The humoral immune response is crucial for protection against flavivirus infection and disease (19). Antibodies can prevent infection by interference with functions mediated by viral surface proteins, such as receptor attachment, virus internalization, and membrane fusion. Indeed, the E glycoprotein is the principal antigen that elicits neutralizing antibodies against flaviviruses. Many of the neutralizing epitopes are located in DIII of E and in close proximity to the conserved fusion loop in DII (20–28). E16 is a strongly neutralizing mAb against WNV that recognizes four polypeptide loops at the tip of DIII and inhibits infection primarily subsequent to cell attachment. The antibody has therapeutic potential as it has been shown to clear WNV infections when administered up to 5 days postinfection (28, 29).
Here, we report a 3D cryo-electron microscopy (EM) image reconstruction that shows that the Fab fragment of E16 uses binding sites on the viral surface as predicted by Nybakken et al. (29). Specifically, Fab E16 does not bind to the DIIIs closest to the fivefold axes. We suggest that E16 neutralizes WNV by sterically interfering with the E rearrangement before fusion on a pathway proposed by Kuhn et al. (5). More weakly or non-neutralizing antibodies bind further from the tip of DIII where they are less likely to inhibit the conformational changes predicted to occur before fusion.
Results and Discussion
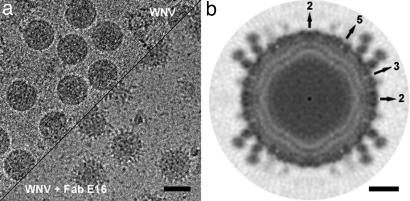
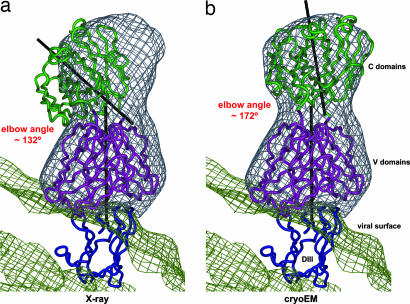
The 3D cryo-EM density map of WNV complexed with the Fab E16 had an estimated resolution of ≈14.5 Å (Figs. 1 and 2). The glycoprotein shell and the two membrane leaflets were clearly resolved. The Fab molecules used only 120 of the 180 possible binding sites on the viral surface, binding to the DIIIs close to the icosahedral threefold axes (DIII-C) and the DIIIs forming the outer circle around the icosahedral fivefolds (DIII-B) (Figs. 2 and 3). No Fab binding was detected at the DIIIs close to the icosahedral fivefold axes (DIII-A), consistent with earlier predictions made from computational docking studies (29) using the x-ray structure of the Fab E16 in complex with DIII and a pseudoatomic model of WNV E. The density related to the variable domains of Fab E16 is about as strong as the density of the glycoprotein shell, suggesting close to 100% occupancy of the 120 binding sites. The density related to the constant regions of Fab E16 is ≈0.6-fold weaker than the variable region, suggesting a flexibility of the elbow angle between variable and constant domains. Indeed, a conformational change in the elbow angle of ≈40° relative to that in the crystal structure of the Fab E16+DIII complex (Fig. 4) shows flexibility, as is often found in antibody structures (30–33). Presumably, the very similar elbow angles in the Fab molecules bound to the independent binding sites DIII-B and DIII-C in the cryo-EM structure represent the lowest energy conformation, whereas the x-ray structure may have a slightly higher energy to achieve better crystal packing.
Fig. 1.
Cryo-EM reconstruction of WNV in complex with the Fab of the neutralizing anti-DIII mAb E16. (a) Cryo-EM micrograph of vitrified WNV particles alone (Left) and complexed with Fab E16 (Right). (Scale bar: 500 Å.) (b) Central cross-section of the 3D image reconstruction of the complex viewed down an icosahedral twofold axis. The positions of icosahedral twofold, threefold, and fivefold axes are indicated. (Scale bar: 100 Å.)
Fig. 2.
Stereoview showing the surface rendering of the 3D image reconstruction of WNV (green) in complex with Fab E16 (blue) at 14.5-Å resolution, viewed down an icosahedral twofold axis. The black triangles mark an icosahedral asymmetric unit.
Fig. 3.
Asymmetric utilization of binding sites by Fab E16. (a) Arrangement of the E glycoproteins on the viral surface. One icosahedral asymmetric unit is outlined in black. DI, DII, and DIII of each E momomer are colored red, yellow, and blue, respectively. The fusion loop is shown in green. (b) E protein arrangement within a defined icosahedral asymmetric unit. The shadows outline three E monomers. The three independent positions of DIII are labeled DIII-A, DIII-B, and DIII-C. (c) Difference density (gray) between the WNV+Fab E16 complex and WNV superpositioned onto the E protein arrangement, viewed down an icosahedral twofold axis. (d) As in c, but viewed down a fivefold axis of symmetry. Fab E16 binds to DIII-B and DIII-C, but not to the fivefold-proximal position DIII-A because of steric hindrance.
Fig. 4.
Fit of Fab E16 into the cryo-EM difference density (gray). (a) Crystal structure of the Fab E16+DIII complex shown after superpositioning of the x-ray DIII portion (blue) onto DIII-C of the fitted WNV E protein. The variable domains are shown in magenta; the constant domains are in green. The black axes represent the pseudodyads between light and heavy chain for the variable and the constant parts. (b) As in a, but the constant domains were adjusted to fit to the cryo-EM density, resulting in an increase of the elbow angle by 40°.
Within the limits of the resolution of the map, the fitting of the x-ray coordinates of the WNV E protein and the Fab E16+DIII complex into the cryo-EM density (see Methods) did not indicate any conformational changes of the E structure with respect to uncomplexed WNV (W.Z., B.K., R.J.K., and M.G.R., unpublished results). Thus, there is no evidence of an induced conformational change of the E protein caused by the binding of Fab E16. The absence of Fab binding to the DIIIs close to the fivefold axes of the virion is only possible as a result of the three E protomers in the icosahedral asymmetric unit having nonequivalent environments. In contrast to DIII-B and DIII-C, the binding of Fab E16 to DIII-A is impossible because of steric interference of the potentially bound Fab with a neighboring, symmetry-related DIII-A (Fig. 5).
Fig. 5.
Stereoview showing the steric hindrance between a potentially bound Fab E16 and a neighboring E molecule close to the icosahedral fivefold axis. Part of the Cα backbone of one DIII-A is shown in dark blue with the E16 epitope in green and the corresponding bound Fab molecule in magenta. A severe clash can be observed between the Fab and a symmetry-related DIII-A (blue), verifying that E16 cannot bind to any of the DIII-As.
Flaviviruses enter their host cell by receptor-mediated endocytosis (34, 35). Once in the endosome, a pH-dependent conformational rearrangement of the E glycoprotein associated with a repositioning of DIII and the exposure of a hydrophobic fusion loop lead to membrane fusion during the cell entry process (14, 15). E16 IgG and Fab fragments neutralize WNV infection efficiently, suggesting that antibody bivalency is not required for neutralization (28), but E16 only partially blocks virus attachment to permissive cells (29). Indeed, the viral surface is not completely shielded by the binding of Fab E16, nor would it be by the binding of the whole E16 antibody (Fig. 6). The unoccupied DIII-A domains close to the icosahedral fivefold axes would allow receptor recognition. Similarly, the glycan at Asn-154 on the neighboring DI is also accessible and, therefore, can be targeted by dendritic cell-specific intercellular adhesion molecule 3-grabbing nonintegrin (DC-SIGNR) (36), which has been suggested to be an accessory receptor.
Fig. 6.
A computer-generated model, shown as a stereo diagram, suggests that the binding of the whole E16 antibody to WNV would leave surface areas accessible to putative receptor binding. The antibody density was calculated from the x-ray coordinates of an IgG (PDB ID code 1IGY) after superpositioning one Fab of the IgG on the fitted Fab E16. The WNV surface is shown in green, and Fab E16 is in blue. The unbound Fab portion of the IgG is colored red, and the Fc portion is yellow. The black triangle marks an icosahedral asymmetric unit. A large portion of the WNV surface is accessible, including the glycan at Asn-154 (red arrow). Severe clashes between antibodies bound to DIII-Bs (forming the outer circle around the icosahedral fivefolds) suggest that only some of the possible binding sites can be occupied simultaneously by the whole E16 antibody. Partial occupancy would leave the area close to the fivefold axes accessible to putative receptor molecules.
E16 blocks infection primarily after attachment to the host cell, presumably by stabilizing the prefusion state of the E protein arrangement in the mature virion or otherwise interfering with the transition to the fusogenic state (29). No intermediates of the E reorganization from the mature virion to a fusion-active state have been captured so far. It has been proposed that the pH-triggered dissociation of the E dimers is immediately followed by an outward rotation of DII before a lateral repositioning of E monomers into trimers (14, 15). In a more detailed proposal based on a cryo-EM structure of T = 1 subviral particles of tick-borne encephalitis virus (37), Kuhn et al. (5) suggest a pH-triggered dissociation of interdimeric contacts and reorganization of E dimers to an intermediate particle of classical T = 3 symmetry, with solvent-exposed membrane areas and E homotrimers suitable for the formation of the radially extended postfusion trimers. Caspar and Klug (38) had proposed that proteins that can form hexamers might readily be able to form pentamers with similar intersubunit contacts, giving rise to various triangulation patterns in which the monomeric subunits all would have similar environments. This concept has been amply verified in observing that many viruses can readily assemble into particles with various T numbers that have essentially the same intersubunit contacts in each case. For instance, the normally T = 3 Southern bean mosaic virus virions can also form T = 1 particles with the same quasi-equivalent contacts between subunits (39). Thus, given the structure of the T = 1 dengue particles observed by Ferlenghi et al. (37), it is probable that the dengue E monomers would also be able to assemble into an equivalent T = 3 lattice. Although this T = 3 structure is not the observed structure of the mature virus, it is probable that it could exist under suitable circumstances. This structure is in essence a set of 60 trimers and would thus be a likely intermediate for the formation of the fusion-competent trimers. Furthermore, in this T = 3 structure the viral membrane is largely exposed for fusion with the host cell, making it a likely conformational transition intermediate.
The bound E16 molecules would inhibit virus infection by sterically hindering the conformational rearrangement of the E glycoprotein dimers into trimers, as suggested by Kuhn et al. (5), before forming the fusogenic state (Fig. 7). In addition, the proposed conformational change requires the greatest movement of domains DIII-B and DIII-C where the bound antibodies would have significant impact on inhibiting the transition toward the fusogenic trimeric form. Oliphant et al. (28) have suggested that a dominant binding site for neutralizing antibodies such as E16 is located at the distal lateral tip of DIII of the E protein. This site would be closest to the icosahedral threefold axes in the proposed conformational T = 3 intermediate. Furthermore, non-neutralizing antibodies would be further away from the threefold axes where steric interference between antibodies bound to neighboring E monomers would be less severe and, hence, would not interfere with the changes required to achieve the fusogenic state. Therefore, Fab E16 might be useful to capture an intermediate of the conformational rearrangement of E on the viral surface during endosomal acidification before fusion. This study enables future comparison of binding patterns of neutralizing versus non-neutralizing antibodies and of virus-specific versus flavivirus cross-reactive antibodies.
Fig. 7.
Inhibition of the low pH-triggered conformational rearrangement to a putative prefusion T = 3 structure during flavivirus cell entry. (a) Dengue virus T = 3 particle suggested to be an intermediate in the rearrangement of the E glycoproteins (5). DI, DII, and DIII of each E momomer are colored red, yellow, and blue, respectively. The fusion loop is shown in green. The black triangle marks an icosahedral asymmetric unit. (b) Arrangement of DIIIs at the icosahedral threefold (quasi-sixfold) axis of the T = 3 particle. The shadows outline symmetry-related asymmetric units. DIIIs originating from position DIII-B of the mature virion are colored blue, and DIII-Cs are light blue. (c) Arrangement of the variable domains of the docked Fab E16 at the icosahedral threefold axis of the T = 3 particle. Each of the six Fabs is colored differently. Significant clashes between neighboring Fabs can be observed, potentially inhibiting the fusion pathway. (d) Arrangement of the constant domains of Fab E16 at the icosahedral threefold axis also results in major clashes.
Methods
WNV Propagation and Purification.
BHK21 cells were grown in MEM supplemented with 10% FCS following standard cell culture procedures. Confluent cells were infected with WNV (New York 1999) at a multiplicity of infection of 0.25 in the presence of 2.5% FCS. Cell culture supernatant was harvested 35 h after infection and concentrated 10-fold with Amicon centrifugal filters (Millipore, Billerica, MA). The virus was purified by sedimentation through a 20% sucrose cushion, followed by density gradient centrifugation using a 10–35% tartrate step gradient (125,000 × g for 2 h at 4°C). The virus fraction was pulled from the gradient, transferred into NTE buffer (12 mM Tris·HCl, pH 8/120 mM NaCl/1 mM EDTA) and concentrated using Amicon centrifugal filters. The protein concentration and purity of the final virus preparations were estimated by SDS gel electrophoresis with Coomassie blue staining.
Antibody Production and Purification of Fab Fragments.
The humanized mAb E16 (subtype IgG2b) was generated and purified as described (28). Fab fragments were purified after papain digestion of the antibody by a protein A column. The flow-through was then further purified over a Superdex 75 16/60 gel filtration column (GE Healthcare/Amersham Pharmacia Biosciences, Piscataway, NJ).
Complex Formation, Cryo-EM, and 3D Image Reconstruction.
Purified WNV particles were incubated with Fab in the presence of 100 mM NaCl at 4°C overnight using a ratio of about five Fab fragments per E molecule. Micrographs of the frozen-hydrated samples were recorded on Kodak (Rochester, NY) SO-163 films with a CM300 FEG transmission electron microscope (Philips, Eindhoven, The Netherlands) (Fig. 1a). Images were taken at a nominal magnification of ×45,000 and a total electron dose of ≈22 e−/Å2. The cryo-EM micrographs were digitized on a Nikon (Tokyo, Japan) 9000 scanner with a 6.35-μm step size. Subsequently, sets of four pixels were averaged, resulting in a sampling of the specimen images at 2.82-Å intervals.
A cryo-EM density map of WNV was used to initialize the 3D reconstruction. The orientations and origins of all of the particles were determined by comparing the contrast transfer function-corrected particle images against reference projections of the current EM reconstructed map by using the programs PFTSEARCH (40) and POR (41). The procedure was repeated at progressively higher spatial frequencies until no further increase in the correlation coefficients could be obtained. To improve the reliability of the orientation refinement, the image data were band-pass-filtered to reduce low- and high-frequency noise. The resolution of the resulting map was estimated by comparing structure factors of the virus shell computed from two independent half-data sets (Table 1; EM Databank accession no. EMD-1234). For the final 3D reconstruction, data were included to a resolution where the correlation between the two independent data sets was 0.3 (Fig. 2). A difference map between the WNV–Fab complex and WNV on its own was calculated after scaling of the densities to optimize the radial dimensions and average density levels (Fig. 3). The magnifications of the cryo-EM maps were standardized to a map calculated from dengue virus model coordinates (Protein Data Bank ID code 1THD), resulting in a final pixel separation of 2.75 Å.
Table 1.
Image data for the cryo-EM reconstruction of the WNV + Fab E16 complex
| No. of micrographs | 78 |
| Defocus level range, μm | 3.88–1.32 |
| No. of particles selected from micrographs | 5.427 |
| No. of particles used for reconstruction | 3,567 |
| Final resolution, Å | 14.5* (12.8†) |
*Resolution at a Fourier shell correlation coefficient of 0.5 between independent half data sets.
†Resolution at a Fourier shell correlation coefficient of 0.3 between independent half data sets.
Fitting of X-Ray Coordinates into the WNV–Fab Cryo-EM Density.
Initially, the crystallographically determined coordinates of the WNV E ectodomains (PDB accession no. 2HG0) (G.E.N., B. Chen, M.S.D., and D.H.F., unpublished work) were fitted into the cryo-EM density of the complex constrained by icosahedral symmetry using the program EMfit, version SR5 (42). The results established that the general arrangement of the WNV E monomers in the complex is essentially the same as for dengue virus within the resolution of the map, indicating that the bound Fab molecules did not significantly change the conformation of the E proteins.
Each of the three WNV E monomers in the icosahedral asymmetric unit was divided into two rigid bodies, DI+III (residues 1–51, 133–195, and 283–400) and DII (residues other than DI+III) for separate, independent fitting, as was also described for dengue virus (9). The position and orientation of each rigid body was determined one at a time by using a complete 3D angular search and assuming an initial center of gravity derived from the analogous dengue virus E structure, followed by rotational and translational refinement (Table 2). The density corresponding to the best fit of one molecule was set to zero before fitting the next molecule to avoid steric clashes between sequentially fitted fragments. Domain DI+III was fitted by using a maximum distance restraint of 10 Å between the termini of strands connecting DII and DI. Although the WNV E DII structure fitted into the cryo-EM density, significant clashes resulted between the symmetry-related loops close to the dimer axes. These clashes do not occur in the crystal structure, because in contrast to dengue virus E protein dimer (8, 9), WNV E crystallized as a monomer (G.E.N., B. Chen, M.S.D., and D.H.F., unpublished work). Therefore, residues 256–260 within the loop connecting the β-strands i and k of DII (see ref. 7 for loop nomenclature) were deleted before the final fitting.
Table 2.
Statistics for fitting of Cα atoms only
| Structural unit | Position in asymmetric unit | Sumf | Clash | −Den | Avgds |
|---|---|---|---|---|---|
| DI + III | A | 3.7 | 9.5 | 0.9 | 7.5 |
| B | 3.6 | 0.0 | 0.4 | 8.2 | |
| C | 3.7 | 2.6 | 0.4 | 6.4 | |
| DII | A | 3.8 | 0.0 | 0.0 | – |
| B | 3.9 | 0.0 | 0.0 | – | |
| C | 3.7 | 0.0 | 0.0 | – | |
| 16V + DI + III | B | 3.2 | 0.0 | 1.7 | – |
| C | 3.5 | 0.0 | 0.0 | – | |
| 16C | B | 2.5 | 0.0 | 0.5 | 7.3 |
| C | 2.4 | 0.0 | 1.5 | 5.5 |
For designation of molecule position in icosahedral asymmetric unit see Fig. 3. For definition of terms see program EMfit (42). SumF (%) is defined as the average value of density for all Cα atomic positions normalized by the rms deviation from the mean of the map. Clash represents the percentage of atoms in one subunit that have steric clashes with symmetry-related subunits. −Den refers to the percentage of atoms that are positioned in negative density. Avgds (Å) is the average distance between restraining and target positions.
Fab E16 was bound to only two of the three available E DIIIs per icosahedral asymmetric unit (Fig. 3), using the DIIIs close to the icosahedral threefold axes (DIII-C) and the DIIIs forming the outer circle around the icosahedral fivefolds (DIII-B). However, no Fabs were bound to the DIIIs close to the icosahedral fivefold axes forming the inner circle about the fivefold axes (DIII-A). When the DIII of the FabE16+DIII crystal structure (PDB accession no. 1ZTX) was superpositioned onto a DIII-C, the constant domains of the Fab were out of the cryo-EM density (Fig. 4a). Alternatively, when the Fab portion (Fab E16; residues L1-L212 and H1-H228) of the crystal structure was fitted into the difference cryo-EM map, the DIII portion did not superimpose correctly onto DIII-C of the WNV structure. Thus, either the elbow angle between the variable and the constant domains or the interaction of the Fab with domain III was different in the cryo-EM reconstruction and the crystal structure. Therefore, the variable domain by itself (16V; residues L1-L107 and H1-H113) was fitted into the difference map, resulting in a positional relationship of DIII and variable domains closely similar to that observed in the crystal structure. The structure was completed by fitting the constant domains (16C; residues L108-L212 and H114-H228) into the density using a distance restraint of 10 Å between the C termini of 16V and the N termini of 16C. Hence, the elbow angle is different in the cryo-EM reconstruction and the crystal structure, changing by ≈40° from 132° in the crystal structure to ≈172° at site DIII-C and 168° at DIII-B in the cryo-EM structure (Fig. 4). Accordingly, the rigid body 16V+DI+DIII was constructed for further improvement of the fitting. The procedures described above were repeated for the Fab bound to DIII-B, giving very similar results, which verified the accuracy of the fitting at DIII-C.
Figure Preparation.
Figures were created by using the programs DINO (www.dino3d.org) and POV-Ray (www.povray.org).
Acknowledgments
We thank Chuan Xiao and Marc Morais for support with computer programs; Stephen Burke for preparation of the Fab fragments; and Sharon Wilder, Sheryl Kelly, and Cheryl Towell for help in the preparation of the manuscript. This work was supported by National Institutes of Health Grant AI55672 (to R.J.K. and M.G.R.), National Institutes of Health Grant A1061373 (to M.S.D.), and grants from the Pediatric Dengue Vaccine Initiative (to R.J.K., M.G.R., D.H.F., and M.S.D.).
Glossary
Abbreviations
- WNV
West Nile virus
- DI
domain I
- DII
domain II
- DIII
domain III
- EM
electron microscopy.
Footnotes
Conflict of interest statement: No conflicts declared.
This paper was submitted directly (Track II) to the PNAS office.
Data deposition: The EM density map reported in this paper has been deposited in the EM Databank (accession no. EMD-1234).
References
- 1.Lanciotti R. S., Roehrig J. T., Deubel V., Smith J., Parker M., Steele K., Crise B., Volpe K. E., Crabtree M. B., Scherret J. H., et al. Science. 1999;286:2333–2337. doi: 10.1126/science.286.5448.2333. [DOI] [PubMed] [Google Scholar]
- 2.Gould L. H., Fikrig E. J. Clin. Invest. 2004;113:1102–1107. doi: 10.1172/JCI21623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hayes E. B., Gubler D. J. Annu. Rev. Med. 2006;57:181–194. doi: 10.1146/annurev.med.57.121304.131418. [DOI] [PubMed] [Google Scholar]
- 4.Hall R. A., Scherret J. H., Mackenzie J. S. Ann. N. Y. Acad. Sci. 2001;951:153–160. [PubMed] [Google Scholar]
- 5.Kuhn R. J., Zhang W., Rossmann M. G., Pletnev S. V., Corver J., Lenches E., Jones C. T., Mukhopadhyay S., Chipman P. R., Strauss E. G., et al. Cell. 2002;108:717–725. doi: 10.1016/s0092-8674(02)00660-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mukhopadhyay S., Kim B. S., Chipman P. R., Rossmann M. G., Kuhn R. J. Science. 2003;302:248. doi: 10.1126/science.1089316. [DOI] [PubMed] [Google Scholar]
- 7.Rey F. A., Heinz F. X., Mandl C., Kunz C., Harrison S. C. Nature. 1995;375:291–298. doi: 10.1038/375291a0. [DOI] [PubMed] [Google Scholar]
- 8.Modis Y., Ogata S., Clements D., Harrison S. C. Proc. Natl. Acad. Sci. USA. 2003;100:6986–6991. doi: 10.1073/pnas.0832193100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang Y., Zhang W., Ogata S., Clements D., Strauss J. H., Baker T. S., Kuhn R. J., Rossmann M. G. Structure (London) 2004;12:1607–1618. doi: 10.1016/j.str.2004.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pokidysheva E., Zhang Y., Battisti A. J., Bator-Kelly C. M., Chipman P. R., Xiao C., Gregorio G. G., Hendrickson W. A., Kuhn R. J., Rossmann M. G. Cell. 2006;124:485–493. doi: 10.1016/j.cell.2005.11.042. [DOI] [PubMed] [Google Scholar]
- 11.Allison S. L., Schalich J., Stiasny K., Mandl C. W., Heinz F. X. J. Virol. 2001;75:4268–4275. doi: 10.1128/JVI.75.9.4268-4275.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bhardwaj S., Holbrook M., Shope R. E., Barrett A. D., Watowich S. J. J. Virol. 2001;75:4002–4007. doi: 10.1128/JVI.75.8.4002-4007.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Heinz F. X., Allison S. L. Adv. Virus Res. 2003;59:63–97. doi: 10.1016/s0065-3527(03)59003-0. [DOI] [PubMed] [Google Scholar]
- 14.Bressanelli S., Stiasny K., Allison S. L., Stura E. A., Duquerroy S., Lescar J., Heinz F. X., Rey F. A. EMBO J. 2004;23:728–738. doi: 10.1038/sj.emboj.7600064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Modis Y., Ogata S., Clements D., Harrison S. C. Nature. 2004;427:313–319. doi: 10.1038/nature02165. [DOI] [PubMed] [Google Scholar]
- 16.Hung J. J., Hsieh M. T., Young M. J., Kao C. L., King C. C., Chang W. J. Virol. 2004;78:378–388. doi: 10.1128/JVI.78.1.378-388.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chu J. J., Rajamanonmani R., Li J., Bhuvanakantham R., Lescar J., Ng M. L. J. Gen. Virol. 2005;86:405–412. doi: 10.1099/vir.0.80411-0. [DOI] [PubMed] [Google Scholar]
- 18.Lee J. W., Chu J. J., Ng M. L. J. Biol. Chem. 2006;281:1352–1360. doi: 10.1074/jbc.M506614200. [DOI] [PubMed] [Google Scholar]
- 19.Diamond M. S., Shrestha B., Mehlhop E., Sitati E., Engle M. Viral Immunol. 2003;16:259–278. doi: 10.1089/088282403322396082. [DOI] [PubMed] [Google Scholar]
- 20.Roehrig J. T. Adv. Virus Res. 2003;59:141–175. doi: 10.1016/s0065-3527(03)59005-4. [DOI] [PubMed] [Google Scholar]
- 21.Hiramatsu K., Tadano M., Men R., Lai C. J. Virology. 1996;224:437–445. doi: 10.1006/viro.1996.0550. [DOI] [PubMed] [Google Scholar]
- 22.Beasley D. W., Barrett A. D. J. Virol. 2002;76:13097–13100. doi: 10.1128/JVI.76.24.13097-13100.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wu K. P., Wu C. W., Tsao Y. P., Kuo T. W., Lou Y. C., Lin C. W., Wu S. C., Cheng J. W. J. Biol. Chem. 2003;278:46007–46013. doi: 10.1074/jbc.M307776200. [DOI] [PubMed] [Google Scholar]
- 24.Crill W. D., Chang G. J. J. Virol. 2004;78:13975–13986. doi: 10.1128/JVI.78.24.13975-13986.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Volk D. E., Beasley D. W., Kallick D. A., Holbrook M. R., Barrett A. D., Gorenstein D. G. J. Biol. Chem. 2004;279:38755–38761. doi: 10.1074/jbc.M402385200. [DOI] [PubMed] [Google Scholar]
- 26.Goncalvez A. P., Purcell R. H., Lai C. J. J. Virol. 2004;78:12919–12928. doi: 10.1128/JVI.78.23.12919-12928.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sanchez M. D., Pierson T. C., McAllister D., Hanna S. L., Puffer B. A., Valentine L. E., Murtadha M. M., Hoxie J. A., Doms R. W. Virology. 2005;336:70–82. doi: 10.1016/j.virol.2005.02.020. [DOI] [PubMed] [Google Scholar]
- 28.Oliphant T., Engle M., Nybakken G. E., Doane C., Johnson S., Huang L., Gorlatov S., Mehlhop E., Marri A., Chung K. M., et al. Nat. Med. 2005;11:522–530. doi: 10.1038/nm1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nybakken G. E., Oliphant T., Johnson S., Burke S., Diamond M. S., Fremont D. H. Nature. 2005;437:764–769. doi: 10.1038/nature03956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Harris L. J., Larson S. B., Hasel K. W., Day J., Greenwood A., McPherson A. Nature. 1992;360:369–372. doi: 10.1038/360369a0. [DOI] [PubMed] [Google Scholar]
- 31.Guddat L. W., Shan L., Fan Z. C., Andersen K. N., Rosauer R., Linthicum D. S., Edmundson A. B. FASEB J. 1995;9:101–106. doi: 10.1096/fasebj.9.1.7821748. [DOI] [PubMed] [Google Scholar]
- 32.Saphire E. O., Stanfield R. L., Crispin M. D., Parren P. W., Rudd P. M., Dwek R. A., Burton D. R., Wilson I. A. J. Mol. Biol. 2002;319:9–18. doi: 10.1016/S0022-2836(02)00244-9. [DOI] [PubMed] [Google Scholar]
- 33.Stanfield R. L., Zemla A., Wilson I. A., Rupp B. J. Mol. Biol. 2006;357:1566–1574. doi: 10.1016/j.jmb.2006.01.023. [DOI] [PubMed] [Google Scholar]
- 34.Chu J. J., Ng M. L. J. Virol. 2004;78:10543–10555. doi: 10.1128/JVI.78.19.10543-10555.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lindenbach B. D., Rice C. M. Adv. Virus Res. 2003;59:23–61. doi: 10.1016/s0065-3527(03)59002-9. [DOI] [PubMed] [Google Scholar]
- 36.Davis C. W., Nguyen H. Y., Hanna S. L., Sanchez M. D., Doms R. W., Pierson T. C. J. Virol. 2006;80:1290–1301. doi: 10.1128/JVI.80.3.1290-1301.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ferlenghi I., Clarke M., Ruttan T., Allison S. L., Schalich J., Heinz F. X., Harrison S. C., Rey F. A., Fuller S. D. Mol. Cell. 2001;7:593–602. doi: 10.1016/s1097-2765(01)00206-4. [DOI] [PubMed] [Google Scholar]
- 38.Caspar D. L., Klug A. Cold Spring Harbor Symp. Quant. Biol. 1962;27:1–24. doi: 10.1101/sqb.1962.027.001.005. [DOI] [PubMed] [Google Scholar]
- 39.Erickson J. W., Silva A. M., Murthy M. R., Fita I., Rossmann M. G. Science. 1985;229:625–629. doi: 10.1126/science.4023701. [DOI] [PubMed] [Google Scholar]
- 40.Baker T. S., Cheng R. H. J. Struct. Biol. 1996;116:120–130. doi: 10.1006/jsbi.1996.0020. [DOI] [PubMed] [Google Scholar]
- 41.Ji Y., Marinescu D. C., Zhang W., Zhang X., Yan X., Baker T. S. J. Struct. Biol. 2006;154:1–19. doi: 10.1016/j.jsb.2005.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rossmann M. G., Bernal R., Pletnev S. V. J. Struct. Biol. 2001;136:190–200. doi: 10.1006/jsbi.2002.4435. [DOI] [PubMed] [Google Scholar]