Abstract
Here, we study the intricate relationship between gut microbiota and host cometabolic phenotypes associated with dietary-induced impaired glucose homeostasis and nonalcoholic fatty liver disease (NAFLD) in a mouse strain (129S6) known to be susceptible to these disease traits, using plasma and urine metabotyping, achieved by 1H NMR spectroscopy. Multivariate statistical modeling of the spectra shows that the genetic predisposition of the 129S6 mouse to impaired glucose homeostasis and NAFLD is associated with disruptions of choline metabolism, i.e., low circulating levels of plasma phosphatidylcholine and high urinary excretion of methylamines (dimethylamine, trimethylamine, and trimethylamine-N-oxide), coprocessed by symbiotic gut microbiota and mammalian enzyme systems. Conversion of choline into methylamines by microbiota in strain 129S6 on a high-fat diet reduces the bioavailability of choline and mimics the effect of choline-deficient diets, causing NAFLD. These data also indicate that gut microbiota may play an active role in the development of insulin resistance.
Keywords: metabonomics, NMR, nonalcoholic fatty liver disease, nutritional genomics, metabolic syndrome
Highly complex animals such as mammals can be considered as “superorganisms” with a karyome, a chondriome, and a microbiome (1), resulting from a coevolutionary symbiotic ecosystem of diverse intestinal microbiota interacting metabolically with the host (2). Recent molecular analyses of human microbiota 16s ribosomal DNA sequences revealed a majority of uncultivated or unknown species with a strong degree of interindividual diversity (3, 4). Also, some of the molecular foundations of beneficial symbiotic host–bacteria relationships in the gut were revealed by colonization of germ-free mice with known microbes and by comparisons of the genomes of members of the intestinal microbiota (5). For instance, Bacteroides thetaiotaomicron, a dominant member of normal distal intestinal microbiota, hydrolyzes otherwise indigestible dietary polysaccharides, thus supplying the host with 10–15% of calorific requirement (6). Gut Lactobacillus spp. are also responsible for a significant proportion of bile acid deconjugation, a process that efficiently reduces lipid absorption in the gut (7). Such symbiotic relationships are the result of coevolution and operate at the genome, proteome, and metabolome levels (6, 8).
Insulin resistance (IR) is central to a cluster of frequent and increasingly prevalent pathologies, including type 2 diabetes mellitus, central obesity, hypertension hepatic steatosis, and dyslipidemia (9). IR contributes to major causes of morbidity and mortality worldwide (10). Epidemiological and genetic studies in human and animal models have demonstrated the importance of both genetic and environmental factors in the etiology of IR (9): Dietary variation and intervention, in particular, have a strong influence on the development of IR. Nonalcoholic fatty liver disease (NAFLD), is the most frequent liver condition associated with IR (11). It is associated with hepatic IR and characterized by hepatic accumulation of triglycerides, or steatosis. Although the causes of human NAFLD are not understood, it has been shown in animal models that choline-deficient diets are associated with NAFLD (12).
The critical involvement of the gut microbiota in biological processes controlling host metabolic regulations (13), including those involved in insulin sensitivity and caloric recovery from the diet, is emerging from recent studies (14): Conventionalized animals have 40% more body fat than germ-free animals. Moreover, diet is known to modulate gut-microbial composition (15), and obesity correlates with variation in the distribution of Bacteroidetes and Firmicutes in mice (16). Hence, symbiotic bacterial contributions to IR and NAFLD should not be overlooked.
Novel approaches are emerging to measure and model metabolism in diverse compartments in interacting multicellular systems that also involve symbiotic microorganisms (2). Alongside functional genomic profiling methods such as transcriptomics and proteomics, metabonomics is a metabolic systems-biology approach that can be encapsulated as “understanding the metabolic responses of living systems to pathophysiological stimuli by using multivariate statistical analysis of biological NMR spectroscopic data” (17, 18).1H NMR spectroscopy of biofluids has long been established as a method for profiling abnormal biochemistry and, indeed, was applied to describe diabetic and hyperglyceridaemic phenotypes >20 years ago (19). We have recently applied metabonomics to characterize the intergenome interactions in mice with symbiotic gut microflora and parasitic Schistosoma mansoni infection in mice (20). We have also monitored the gut-microbial metabolite variation in urine from acclimatizing formerly germ-free rats (21).
In this study, we have tested the effects of dietary changes, i.e., switching from a 5% control low-fat diet (LFD) to a 40% high-fat diet (HFD), on plasma and urine metabolic 1H NMR profiles in inbred mouse strain 129S6, documented for its susceptibility to IR or NAFLD (22), and in BALBc strain, which exhibits evidence of resistance to these phenotypes. We characterize here the metabolic profiles related to the cometabolome homeostatic variation (23) and show that microbial metabolism strongly contributes to a NAFLD metabotype, i.e., a quantitative combination of several metabolites, related to IR.
Results
Overview of the Pathophysiological Effects of Fat-Feeding.
We present here background data primarily focused on glucose tolerance and glucose-induced insulin secretion in vivo as well as structural and biochemical markers of hepatic dysfunction.
Glucose Homeostasis and Insulin Secretion.
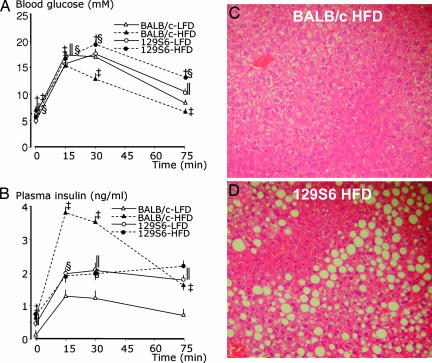
The effects of HFD in BALB/c and 129S6 mice on glucose tolerance and glucose-stimulated insulin secretion in vivo were assessed by glucose tolerance tests (GTTs) (Fig. 1A and B). On the LFD, the general pattern of glycemic response to the glucose challenge was similar in the two strains (Fig. 1A). On the HFD, both strains develop fasting hyperglycemia. Fat-feeding induces marked glucose intolerance in 129S6, as reflected by sustained hyperglycemia throughout the duration of the GTT in HFD-fed mice when compared with LFD-fed mice (Fig. 1A). In contrast, in BALB/c mice, glucose tolerance is markedly improved by fat-feeding, as reflected by significantly lower plasma glucose values in HFD-fed mice than LFD-fed mice throughout the GTT.
Fig. 1.
Pathophysiology of the response of BALB/c and 129S6 mice to prolonged fat-feeding. Effect of glucose injection on blood glucose (A) and plasma insulin (B) concentrations in BALB/c and 129S6 mice fed an LFD or HFD. H&E-stained liver sections from 5-month-old BALB/c (C) and 129S6 (D) mice on HFD, showing micro- and macrovesicular steatosis in 129S6 mice (magnification, ×20). Plasma glucose and insulin values were obtained from >53 (BALB/c-LFD), 34 (BALB/c-HFD), and 40 (129S6-LFD, 129S6-HFD) mice. Significant differences (P < 0.05) between HFD-fed and LFD-fed 129S6 mice (†), between HFD-fed and LFD-fed BALB/c mice (‡), between LFD-fed 129S6 and BALB/c mice (‖), and between HFD-fed 129S6 and BALB/c mice (§) are shown.
Fasting insulinemia was not significantly different between the strains on LFDs, but, in response to glucose, 129S6 mice secrete more insulin during the GTT than BALB/c mice (Fig. 1B). This apparent increased insulin-secretion capacity in 129S6 when compared with BALB/c does not result in improved glucose tolerance (Fig. 1A), suggesting a relative reduction of the biological action of insulin in 129S6 mice compared with BALB/c mice. In BALB/c mice, fat-feeding induces a strongly significant enhancement of glucose-stimulated insulin secretion when compared with the LFD-fed group (Fig. 1B), which may account for concomitant improved glucose tolerance by HFD in this strain (Fig. 1A). In contrast, in 129S6, insulin-secretion pattern and capacity are not significantly altered by HFD, indicating that glucose intolerance in HFD-fed 129S6 mice develops as the result of insulin resistance rather than insulin-secretion deficiency (Fig. 1 A and B).
Plasma Lipid Profiles.
To characterize dyslipidemia, we applied standard clinical chemistry protocols to compare the concentration of plasma lipids, including triglycerides (TG) and total, high-density lipoprotein (HDL), and low-density lipoprotein (LDL) cholesterol, in the four mouse groups (A.T., unpublished work). On LFD, the two strains show similar levels of plasma lipids, and prolonged HFD feeding induced similar hypercholesterolemia in both strains (Fig. 4 A–D, which is published as supporting information on the PNAS web site).
Liver Histopathology and Dysfunction.
After prolonged high-fat feeding, 129S6 mice develop micro- and macrovesicular steatosis, as evidenced by the accumulation of fat droplets in the liver (Fig. 1D), whereas liver histology remained unchanged in BALB/c mice (Fig. 1C). To further characterize steatosis, we assayed hepatic TG (one of the major storage forms of lipids in liver) and circulating levels of aspartate aminotransferase (AST) and alanine aminotransferase (ALT) (markers of hepatic dysfunction). Liver TG are significantly increased (3.3-fold) in fat-fed 129S6 mice (Fig. 4E). The levels of both AST and ALT are higher in 129S6 compared with BALB/c mice on LFD. Fat-feeding induces a significant increase in ALT and AST in both strains that is more prominent in 129S6 than BALB/c mice (Fig. 1 F and G).
Body Weight Follow-Up.
To characterize obesity, we monitored body weight at different ages. On LFDs, body weight is similar in 129S6 and BALB/c mice at 2, 3, and 5 months of age. In 129S6 mice, body weight is significantly increased after 3, 7, and 15 weeks of HFD when compared with age- and strain-matched LFD-fed mice, whereas it remains similar in LFD- and HFD-fed BALB/c mice (data not shown).
Overall, our observations provide confirmatory evidence of the strong susceptibility of 129S6 mice to NAFLD, impaired glucose tolerance, dyslipidemia, and obesity in response to fat-feeding as observed by Biddinger et al. (22) and provide evidence of resistance to these pathologies in BALB/c mice.
Metabolic Profiling by 1H NMR Spectroscopy at 4 Months After HFD Induction.
The chronic effects of the HFD induction on urinary and plasma metabolic profiles at 4 months after induction for 129S6 and BALB/c mouse strains are illustrated in Fig. 2 and Table 1. As shown in Fig. 2A, a typical 600-MHz 1H NMR spectrum of one HFD-fed 129S6 mouse plasma characterizes common markers of insulin resistance: (CH2)n and CH3 resonances from components of lipoproteins, e.g., cholesterol esters, TG, and phospholipids. A peak from the N-methyl group from phosphatidylcholine (PC) can be observed. Low-molecular-mass metabolites, e.g., lactate, alanine, and glucose, are also present (Fig. 2A).
Fig. 2.
Plasma and urine metabolic profiling by 1H NMR spectroscopy of the response of BALB/c and 129S6 mice to dietary intervention at 5 months of age. Plasma (A) and urine (B) 600-MHz 1H NMR spectra from typical 5-month-old 129S6 mice on HFD. OPLSDA score plots for plasma (C) and urine (D) metabolic profiles. HDL, high-density lipoprotein; LDL, low-density lipoprotein; VLDL, very low-density lipoprotein.
Table 1.
1H NMR-derived metabotypes significantly associated with the response of BALB/c and 129S6 mice to dietary intervention at 5 months of age
| Biomarkers OPLSDA coef | δ, ppm | BALB/c LFD | BALB/c HFD | 129S6 LFD | 129S6 HFD |
|---|---|---|---|---|---|
| PC (p) | 3.219 | −0.24 | 0.71 | −0.43 | −0.07 |
| Choline (u) | 3.203 | −0.28 | 0.56 | −0.26 | 0.09 |
| TMA (u) | 2.892 | −0.28 | 0.20 | −0.41 | 0.70 |
| TMAO (u) | 3.2705 | −0.35 | 0.14 | −0.39 | 0.83 |
| DMA (u) | 2.728 | −0.27 | 0.30 | −0.49 | 0.69 |
| MMA (u) | 2.77 | −0.26 | 0.23 | −0.47 | 0.72 |
| DMG (u) | 2.93 | 0.81 | −0.14 | −0.40 | −0.33 |
| Creatine (u) | 3.0405 | 0.69 | −0.42 | 0.06 | −0.51 |
| Glycerate (u) | 4.0975 | −0.52 | −0.50 | 0.81 | 0.09 |
| Gluratate (u) | 1.792 | −0.18 | −0.48 | 0.84 | −0.39 |
| Isovalerate (u) | 2.19 | −0.41 | −0.47 | 0.88 | −0.17 |
| Pyruvate (u) | 2.347 | −0.15 | 0.81 | −0.50 | 0.00 |
The OPLSDA model coefficients are listed for each of the models reported in Fig. 2. For each metabolite, we report the chemical shift δ (in parts per million) and the OPLSDA-derived correlation (coef) for each class in the model: BALB/c LFD, BALB/c HFD, 129/S6 LFD, and 129/S6 HFD. A positive (negative) correlation means a higher (lower) intensity of the NMR resonance of the metabolite significantly associated to the corresponding class. (p), plasma; (u), urine.
A typical 600-MHz 1H NMR urinary spectrum from a NAFLD-sensitive 129S6 mouse on the HFD is illustrated in Fig. 2B, showing contributions of a wide range of low-molecular-mass metabolites (<1 kDa) from both mammalian metabolism and associated gut-microbial systems (24, 25). The urinary 1H NMR spectrum of this 129S6 mouse on HFD is dominated by microbiota-derived methylamines: dimethylamine, trimethylamine (TMA), and trimethylamine-N-oxide (TMAO). We have shown that these metabolites are derived only from symbiotic bacterial metabolism and not from mammalian metabolism in mice (26). Other gut microbiota-derived metabolites include formate and hippurate. Mammalian metabolites such as creatinine, creatine, and taurine are also excreted as well as intermediary metabolites, such as pyruvate, citrate, oxaloacetate, succinate, lactate, glycerate, 2-oxoisocaproate, 2-oxobutyrate, isovalerate, acetate, and acetotacetate (27) (Fig. 2B).
Data Analysis.
Plasma metabolic profiling provides an insight on lipid and energy metabolism, whereas urine metabolic profiling provides a complementary description of intermediary metabolism (Fig. 2 A and B). These complementary data sets are then used to build orthogonal partial least-squares-discriminant analysis (OPLSDA) (28) models, focusing on the differences among the four experimental groups, i.e., BALB/c LFD, BALB/c HFD, 129S6 LFD, and 129S6 HFD, by using only three latent variables or discriminant scores (Fig. 2 C and D).
Plasma Metabonomic Model.
In the plasma OPLSDA model (Fig. 2C and Table 1), it is possible to differentiate the effects of both genetic background (strain) and environmental manipulation (diet) on the score plot: The main effect of component T1 is to discriminate the diet induction, whereas T2 discriminates the genetic background and T3 the specific response of 129S6 to HFD induction (Fig. 2C). The coefficients characterizing each experimental group used to build the OPLSDA model are represented as pseudospectra (Fig. 5, which is published as supporting information on the PNAS web site). On the LFD, both BALB/c (Fig. 5A) and 129S6 (Fig. 5C) plasma metabolic profiles are quite similar, with negative coefficients for TG and PC resonances. This finding indicates that the two strains on the LFD have low TG and low PC levels when compared with the HFD-matched animals. By contrast, the plasma metabolic response is different in HFD-fed strains. The plasma of BALB/c mice on HFD is characterized by high TG and PC levels (Table 1). This hyperlipidemia is linked to the handling of the HFD challenge (Fig. 5B). However, 129S6 mice on the HFD show dyslipidemia. We also observed increased glucose signals in the NMR profiles significantly associated with fat-fed 129S6 mice (such correlation is directly relevant to impaired glucose homeostasis assessed in this group by glucose tolerance testing) as well as pyruvate and TMAO and decreased PC (Fig. 5D). The metabolic fate of dietary choline is monitored by urine metabolic profiling.
Urine Metabonomic Model.
The urinary OPLSDA model discriminates the four groups (Fig. 2D and Table 1): The major effect on T1 discriminates the HFD induction, whereas T2 and T3 describe the response of each strain to the HFD induction. Urine samples from the reference strain BALB/c on the LFD show higher excretion of creatine and N,N-dimethylglycine compared with the other three groups (Fig. 6A, which is published as supporting information on the PNAS web site). On the HFD, BALB/c mice predominantly demonstrate higher excretion of pyruvate (Fig. 6B). However, 129S6 behaves differently on the LFD, as an increased excretion of glycerate, isovalerate, and glutarate is observed (Table 1 and Fig. 6C). In fact, glycerate, isovalerate, and glutarate are excreted by strain 129S6 only. Glyceric aciduria in 129S6 mice on LFD refers to excretion of a glycolysis intermediate and suggests a genetic predisposition toward an impaired glycolysis of 129S6 mice (29). In striking contrast to BALB/c, 129S6 mice on the HFD excrete mainly methylamines (dimethylamine, TMA, and TMAO), which are choline derivatives produced only by microbiota (26) (Table 1 and Fig. 6D).
Short-Term Metabolic Signatures Linked to HFD Induction.
We have also monitored the short-term urinary metabolic response of 129S6 and BALB/c mice to the HFD challenge by daily urine sampling during 1 week before and after the diet induction (Fig. 7, which is published as supporting information on the PNAS web site). 1H NMR urinary metabolic profiling shows that both strains react immediately to the HFD switch with a substantial excretion of methylamines, but this finding is more dramatic in 129S6 mice.
Characterization of the Link Between Choline Bioavailability and NAFLD.
We further characterized the link between NAFLD and choline bioavailability by: (i) quantifying the levels of circulating total plasma choline and plasma TG to show an imbalance between total choline and plasma TG in 129S6 (Fig. 8, which is published as supporting information on the PNAS web site) and (ii) showing that quantitative variation in dietary choline induces an inverse quantitative variation in liver fat content in 129S6 mice (Fig. 9, which is published as supporting information on the PNAS web site).
Overall, the results derived from metabolic profiling monitor mainly present classical markers of insulin resistance, i.e., lipidemia, glycemia, and phospholipids (PC), but also gut microbiota biomarkers. These results reveal a marked decrease of PC in the plasma of fat-fed 129S6 strain compared with fat-fed BALB/c mice. This decrease is associated with a substantial increase of exclusively bacterial-origin methylamines in the urine of 129S6 mice.
Discussion
We show in this study a significant association between a specific metabotype, e.g., low plasma phosphatidylcholine (PC) and high urinary methylamines and genetic predisposition to HFD-induced NAFLD in mice. Thus, we confirm and complement the initial description of NAFLD in fat-fed 129S6 mice by Biddinger et al. (22). In fact, methylamines are coprocessed by gut microbiota: We have reported that germ-free mice do not excrete TMA and have shown the fundamental role of microbiota in TMA production from its precursor choline (26). Hence, urinary excretion of methylamines (TMA, TMAO, and dimethylamine) is directly related to gut-microbiota metabolism. Thus, these metabolites can be used as a probe of microbial metabolism of choline in the metabolic cross-talk between host and symbionts. This microbial bypass leads to a reduction of choline bioavailability for the host (compared with the dietary fatty acid uptake in liver induced by HFD), as denoted by lower PC levels (see Fig. 8), and seem to trigger NAFLD in return (12). Such significant association indicates that the altered gut-microbial metabolism of choline plays a role in the development of NAFLD, as detailed below.
The Metabolic Fate of Choline and the NAFLD Metabotype: Low Plasma PC and High Urinary Methylamines.
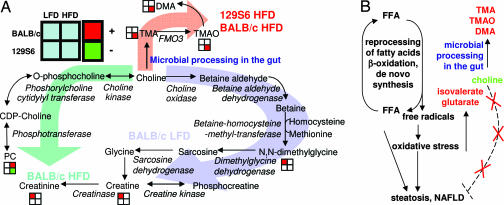
The pathways of choline metabolism involve a complex superorganism host/symbiont molecular cross-talk (Fig. 3A): there are three major pathways using dietary choline, two pure mammalian pathways and a sym-xenobiotic pathway (30). Each one of these pathways has been associated to at least one experimental group: (i) urinary metabolic profiling shows that dietary choline is excreted as NN-dimethylglycine, ultimately leading to the production of creatine and creatinine in BALB/c on the LFD, (ii) choline is also converted to methylamines (TMA, TMAO, and dimethylamine) by gut microbiota in HFD animals in general and 129S6 in particular, (iii) HFD induction leads to a discordant phenotype regarding the circulating PC levels, deriving from choline: Plasma PC levels are significantly lower in 129S6 than in BALB/c mice, even though the diet is supplemented with choline (see Table 2, which is published as supporting information on the PNAS web site). Mice from the 129S6 strain on the HFD are characterized by high methylamines and low plasma PC, and they also develop NAFLD (22). Such distinct metabotypes can be explained as described below.
Fig. 3.
The symbiotic methylamines’ metabolic pathway (A) and their putative mechanism of liver toxicity (B). The OPLS-derived correlation is represented by standard color-coding for each metabolite: red square, positive correlation; green square, negative correlation, meaning a higher (lower) metabolite concentration in the corresponding group. FFA, free fatty acids.
Methylamines and Gut Microbiota.
The first reaction of the methylamine pathway involves conversion of dietary choline into TMA by gut microbiota (31). The microbiota-processed metabolites, or sym-xenobiotic metabolites (30), are absorbed by microvillae and penetrate the systemic circulation through the portal vein between the intestinal tract and liver (32), and TMA is cleared substantially by hepatic first-pass metabolism. This input of xenobiotic or sym-xenobiotic compounds from either symbiotic microbiota or vegetal origin triggers a hepatic detoxification process, involving flavin-containing monooxygenase 3 (FMO3) (32).
Since we showed the gut-microbial origin of TMA and methylamines (26), our more recent results provide indirect evidence that different metabolic activities of microbiota populations exist and that quantitative changes in these microbial metabolic activities are significantly associated with genetically distinct inbred strains resulting in distinct metabolic phenotypes (33, 34). In fact, results from our study suggest the existence of strain-specific and genetically determined selection of gut-microbial metabolism under HFD challenge, based on strain-dependent variation in excretion of methylamines. A possible involvement of gut microbiota in the development of IR-related processes has been suggested by Backhed et al. (14) who reported that the gut microbiota affects energy harvest from the diet and energy storage in the host. It has also been observed that Helicobacter pylori interacts with the appetite-regulating peptides, i.e., leptin and ghrelin (35, 36).
Gut Microbiota Mimic Choline-Deficient Diets.
Choline-deficient diets have been consistently associated with hepatic steatosis, which is reversible by choline i.v. infusion (12). We have also shown that quantitative variation in dietary choline induces an inverse quantitative variation in liver fat content (see Fig. 9). We show here that lower plasma PC levels in strain 129S6 on HFD compared with BALB/c mice can be explained by reduced bioavailability of choline (see Fig. 8) because of conversion of choline into methylamines by gut microbiota, with subsequent urinary excretion. This mechanism thus mimics a choline-deficient diet. This microbiota-related reduced choline bioavailability may result in the inability to synthesize PC necessary for the assembly and secretion of very-low-density lipoprotein (VLDL) (37) and subsequent accumulation of TG in liver. Methylamines also induce hepatotoxicity and hepatocarcinogenicity in rats (38). Indeed, microsomal FMO-detoxification enzymatic systems have been evolutionarily coselected toward the assimilation of biologically active natural compounds involved in biological defense signaling (39). This enzymatic system detoxifies soft nucleophilic functional groups of natural origin, such as alkaloids, with basic side chains, and organic sulfur xenobiotics. Microbiota-derived methylamines, predominantly excreted in urine, share the same metabolic detoxification process and may also share the same toxicity as other soft nucleophiles. Recent metagenomic studies have also shown a strong interaction between gut flora and detoxification of xenobiotics (40).
Putative Mechanisms of Hepatotoxicity.
We propose, in Fig. 3B, an indirect mechanism of hepatotoxicity involving: (i) microbial conversion choline into TMA, thus reducing bioavailability of choline, (ii) influx of fatty acids in the liver, (iii) generation of radical oxidative species via reprocessing of fatty acids and oxidative stress, and (iv) lack of VLDL secretion generating steatosis and NAFLD. In that regard, small variations in circulating levels of choline and PC linked to microbial bypass between the two strains, associated with dietary fatty acid challenge, might trigger irreversible liver damage.
Conclusions
Our observations indicate that gut-microbial metabolism alters the metabolism of the mammalian host. This work strongly supports the idea that complex metabolic disease traits are a product of extended genome (superorganism) perturbations under the influence of an external stressor, in this case, a dietary change. We show that a specific metabotype with low plasma PC and high gut microbiota-mediated urinary excretion of methylamines is associated with the predisposition to impaired glucose homeostasis and NAFLD. Along with mechanisms of suppression of inflammatory response (41, 42) or regulation of fat storage by microbiota (14), we are now able to describe a diet-induced mechanism of steatosis triggered by symbiotic microbiota, another example of “the thin line between gut commensal and pathogen” noted by Gilmore and Ferretti (43). It is also likely that changes in the Western lifestyle induce changes in the gut-microbial ecology. Such changes are likely to affect the nutrigenomic or pharmacometabonomic predisposition (44) toward different energy storage capabilities and pathologies and, in the end, personalized healthcare (23).
Materials and Methods
Animals and Treatment.
Male mice from two inbred strains, BALB/cOxjr (BALB/c) and 129S6/SvEvOxjr (129S6), were bred locally by using a stock originating from The Jackson Laboratory, Bar Harbor, ME. Mice were weaned at 3 weeks, housed in groups of 10–12 animals, maintained under standard breeding conditions, and fed ad libitum a standard carbohydrate and 5% LFD (B & K Universal, Hull, U.K.) from weaning to 5 weeks of age. At 5 weeks of age, one group of mice from each strain was transferred to a 40% HFD (Special Diets Services, Witham, Essex, U.K.), containing 32% lard and 8% corn oil, whereas strain- and age-matched control groups remained on the LFD for the duration of the diet trial. The detailed diet formulation is available in Table 2. Mice were maintained under a 12-h–12-h light–dark regime, with lights off at 7 p.m. All procedures were carried out in accordance with U.K. Home Office guidelines on animal welfare and license conditions and University of Oxford guidelines on animal welfare.
Glucose Tolerance and Insulin-Secretion Tests.
GTTs were performed in mice fed the HFD for 4 months and age-matched controls. After an overnight fast, mice were anesthetized by i.p. injection of sodium pentobarbital (Sagatal; Rhône Mérieux, Athens, GA). Mice were injected i.p. with a single dose of 2 g of glucose per kg of body weight. Blood samples were collected through the tail vein before glucose injection and at 15, 30, and 75 min afterward. Blood glucose concentrations were immediately determined by using a glucose meter (Accucheck; Roche Diagnostics, Lewes, U.K.). Blood samples were centrifuged, and plasma was separated for insulin assays. Plasma insulin concentration was determined by ELISA (Mercodia, Uppsala, Sweden).
Biofluid Sample Collection.
Four days after the glucose tolerance test, 24-h urinary samples (9 a.m. to 9 a.m.) were collected from mice maintained in individual metabolic cages. Urinary samples collected in a solution of 1% (wt/vol) sodium azide were centrifuged to remove solid particles and kept at −80°C until assayed. After an overnight fast, mice were killed by exsanguination. Plasma was separated by centrifugation and stored at −80°C until NMR analysis.
Liver Histology.
Liver biopsies from these fasted mice were fixed in neutral buffered formalin solution (Surgipath Europe, Peterborough, U.K.) for >24 h, dehydrated, embedded in paraffin, and sectioned at 4 μm. Staining of liver sections was carried out with H&E. Results are based on assessment of five or more mice per strain and diet group examined.
1H NMR Spectroscopy.
Mouse urine samples were prepared by using 200 μl of urine mixed with 200 μl of water and 200 μl of 0.1 M phosphate buffer solution (10% 2H2O/H2O vol/vol, with 0.05% sodium 3-trimethylsilyl-(2,2,3,3-2H4)-1-propionate for chemical shift reference at δ-0.0) in 96-well plates for high-throughput flow-injection NMR acquisition. Plasma samples were prepared by using a 100-μl aliquot diluted in 400 μl of a 9 g/liter saline solution (20% 2H2O/H2O vol/vol). Standard 1H NMR spectra were measured on a spectrometer (Bruker, Rheinstetten, Germany) operating at 600.22 MHz 1H frequency, as described (20). The 1H NMR spectra were phase and baseline corrected by using in-house software (T. Ebbels and H. Keun, personal communication) and were imported into Matlab at high resolution as described (45). The regions δ-6.0–5.5 and δ-5.0–4.5 were removed to eliminate baseline effects of imperfect water signal presaturation. Each spectrum was normalized to a constant intensity sum, and each variable was mean centered.
OPLSDA.
The method allows enhanced focus on strain and diet intervention while minimizing other biological/analytical variation. Sample classes were modeled by using the OPLS algorithm. This algorithm derives from the partial least-squares regression method (28). In discriminant analysis version, the method explains the maximum separation between class samples Y (n dummy variables for n classes) by using the NMR data X, by decomposing the covariation matrix (YTX) into n − 1 O-PLS components and several orthogonal signal correction components (46). Further details on standard OPLS implementation in metabonomics have been given in ref. 45. The model coefficients locate the NMR variables associated with a specific class in Y.
Supplementary Material
Acknowledgments
We thank Terry M. Hacker (Medical Research Council, Harwell, U.K.) for helping with liver histology. This study was supported by Wellcome Trust Functional Genomics Initiative Grant 066786 [Biological Atlas of Insulin Resistance (www.bair.org.uk)] and Wellcome Trust Senior Fellowship in Basic Biomedical Science 057733 (to D.G.).
Glossary
Abbreviations
- GTT
glucose tolerance test
- HFD
high-fat diet
- IR
insulin resistance
- LFD
low-fat diet
- NAFLD
nonalcoholic fatty liver disease
- OPLSDA
orthogonal partial least-squares-discriminant analysis
- TG
triglyceride
- PC
phosphatidylcholine
- TMA
trimethylamine
- TMAO
trimethylamine-N-oxide.
Footnotes
Conflict of interest statement: No conflicts declared.
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Lederberg J. Science. 2000;288:287–293. doi: 10.1126/science.288.5464.287. [DOI] [PubMed] [Google Scholar]
- 2.Nicholson J. K., Holmes E., Lindon J. C., Wilson I. D. Nat. Biotechnol. 2004;22:1268–1274. doi: 10.1038/nbt1015. [DOI] [PubMed] [Google Scholar]
- 3.Bik E. M., Eckburg P. B., Gill S. R., Nelson K. E., Purdom E. A., Francois F., Perez-Perez G., Blaser M. J., Relman D. A. Proc. Natl. Acad. Sci. USA. 2006;103:732–737. doi: 10.1073/pnas.0506655103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Eckburg P. B., Bik E. M., Bernstein C. N., Purdom E., Dethlefsen L., Sargent M., Gill S. R., Nelson K. E., Relman D. A. Science. 2005;308:1635–1638. doi: 10.1126/science.1110591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xu J., Gordon J. I. Proc. Natl. Acad. Sci. USA. 2003;100:10452–10459. doi: 10.1073/pnas.1734063100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xu J., Bjursell M. K., Himrod J., Deng S., Carmichael L. K., Chiang H. C., Hooper L. V., Gordon J. I. Science. 2003;299:2074–2076. doi: 10.1126/science.1080029. [DOI] [PubMed] [Google Scholar]
- 7.Narushima S., Ito K., Kuruma K., Uchida K. Lipids. 2000;35:639–644. doi: 10.1007/s11745-000-0568-0. [DOI] [PubMed] [Google Scholar]
- 8.Mutch D. M., Simmering R., Donnicola D., Fotopoulos G., Holzwarth J. A., Williamson G., Corthesy-Theulaz I. Physiol. Genom. 2004;19:22–31. doi: 10.1152/physiolgenomics.00105.2004. [DOI] [PubMed] [Google Scholar]
- 9.Saltiel A. R., Kahn C. R. Nature. 2001;414:799–806. doi: 10.1038/414799a. [DOI] [PubMed] [Google Scholar]
- 10.Zimmet P., Alberti K. G., Shaw J. Nature. 2001;414:782–787. doi: 10.1038/414782a. [DOI] [PubMed] [Google Scholar]
- 11.Day C. P., James O. F. Hepatology. 1998;27:1463–1466. doi: 10.1002/hep.510270601. [DOI] [PubMed] [Google Scholar]
- 12.Buchman A. L., Dubin M. D., Moukarzel A. A., Jenden D. J., Roch M., Rice K. M., Gornbein J., Ament M. E. Hepatology. 1995;22:1399–1403. [PubMed] [Google Scholar]
- 13.Backhed F., Ley R. E., Sonnenburg J. L., Peterson D. A., Gordon J. I. Science. 2005;307:1915–1920. doi: 10.1126/science.1104816. [DOI] [PubMed] [Google Scholar]
- 14.Backhed F., Ding H., Wang T., Hooper L. V., Koh G. Y., Nagy A., Semenkovich C. F., Gordon J. I. Proc. Natl. Acad. Sci. USA. 2004;101:15718–15723. doi: 10.1073/pnas.0407076101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mai V. Nutr. Rev. 2004;62:235–242. doi: 10.1301/nr2004.jun235-242. [DOI] [PubMed] [Google Scholar]
- 16.Ley R. E., Backhed F., Turnbaugh P., Lozupone C. A., Knight R. D., Gordon J. I. Proc. Natl. Acad. Sci. USA. 2005;102:11070–11075. doi: 10.1073/pnas.0504978102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nicholson J. K., Connelly J., Lindon J. C., Holmes E. Nat. Rev. Drug Discov. 2002;1:153–161. doi: 10.1038/nrd728. [DOI] [PubMed] [Google Scholar]
- 18.Nicholson J. K., Lindon J. C., Holmes E. Xenobiotica. 1999;29:1181–1189. doi: 10.1080/004982599238047. [DOI] [PubMed] [Google Scholar]
- 19.Nicholson J. K., O’Flynn M. P., Sadler P. J., Macleod A. F., Juul S. M., Sonksen P. H. Biochem. J. 1984;217:365–375. doi: 10.1042/bj2170365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang Y., Holmes E., Nicholson J. K., Cloarec O., Chollet J., Tanner M., Singer B. H., Utzinger J. Proc. Natl. Acad. Sci. USA. 2004;101:12676–12681. doi: 10.1073/pnas.0404878101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nicholls A. W., Mortishire-Smith R. J., Nicholson J. K. Chem. Res. Toxicol. 2003;16:1395–1404. doi: 10.1021/tx0340293. [DOI] [PubMed] [Google Scholar]
- 22.Biddinger S. B., Almind K., Miyazaki M., Kokkotou E., Ntambi J. M., Kahn C. R. Diabetes. 2005;54:1314–1323. doi: 10.2337/diabetes.54.5.1314. [DOI] [PubMed] [Google Scholar]
- 23.Nicholson J. K., Holmes E., Wilson I. D. Nat. Rev. Microbiol. 2005;3:431–438. doi: 10.1038/nrmicro1152. [DOI] [PubMed] [Google Scholar]
- 24.Nicholson J. K., Sadler P. J., Bales J. R., Juul S. M., MacLeod A. F., Sonksen P. H. Lancet. 1984;2:751–752. doi: 10.1016/s0140-6736(84)92656-4. [DOI] [PubMed] [Google Scholar]
- 25.Bales J. R., Higham D. P., Howe I., Nicholson J. K., Sadler P. J. Clin. Chem. 1984;30:426–432. [PubMed] [Google Scholar]
- 26.al-Waiz M., Mikov M., Mitchell S. C., Smith R. L. Metabolism. 1992;41:135–136. doi: 10.1016/0026-0495(92)90140-6. [DOI] [PubMed] [Google Scholar]
- 27.Lindon J. C., Nicholson J. K., Holmes E., Everett J. R. Concept Magnetic Res. 2000;12:289–320. [Google Scholar]
- 28.Trygg J., Wold S. J. Chemometrics. 2003;17:53–64. [Google Scholar]
- 29.Manuel Keenoy B., Maggetto C., Malaisse W. J. Biochem. Med. Metab. Biol. 1993;50:35–53. doi: 10.1006/bmmb.1993.1045. [DOI] [PubMed] [Google Scholar]
- 30.Nicholson J. K., Wilson I. D. Nat. Rev. Drug Discov. 2003;2:668–676. doi: 10.1038/nrd1157. [DOI] [PubMed] [Google Scholar]
- 31.Zeisel S. H., Wishnok J. S., Blusztajn J. K. J. Pharmacol. Exp. Ther. 1983;225:320–324. [PubMed] [Google Scholar]
- 32.Lang D. H., Yeung C. K., Peter R. M., Ibarra C., Gasser R., Itagaki K., Philpot R. M., Rettie A. E. Biochem. Pharmacol. 1998;56:1005–1012. doi: 10.1016/s0006-2952(98)00218-4. [DOI] [PubMed] [Google Scholar]
- 33.Robosky L. C., Wells D. F., Egnash L. A., Manning M. L., Reily M. D., Robertson D. G. Toxicol. Sci. 2005;87:277–284. doi: 10.1093/toxsci/kfi214. [DOI] [PubMed] [Google Scholar]
- 34.Holmes E., Nicholson J. Toxicol. Sci. 2005;87:1–2. doi: 10.1093/toxsci/kfi259. [DOI] [PubMed] [Google Scholar]
- 35.Nwokolo C. U., Freshwater D. A., O’Hare P., Randeva H. S. Gut. 2003;52:637–640. doi: 10.1136/gut.52.5.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Azuma T., Suto H., Ito Y., Ohtani M., Dojo M., Kuriyama M., Kato T. Gut. 2001;49:324–329. doi: 10.1136/gut.49.3.324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jiang X.-C., Li Z., Liu R., Yang X. P., Pan M., Lagrost L., Fisher E. A., Williams K. J. J. Biol. Chem. 2005;280:18336–18340. doi: 10.1074/jbc.M500007200. [DOI] [PubMed] [Google Scholar]
- 38.Lin J. K., Ho Y. S. Food Chem. Toxicol. 1992;30:695–702. doi: 10.1016/0278-6915(92)90165-h. [DOI] [PubMed] [Google Scholar]
- 39.Ziegler D. M. Trends Pharmacol. Sci. 1990;11:321–324. doi: 10.1016/0165-6147(90)90235-z. [DOI] [PubMed] [Google Scholar]
- 40.Gill S. R., Pop M., Deboy R. T., Eckburg P. B., Turnbaugh P. J., Samuel B. S., Gordon J. I., Relman D. A., Fraser-Liggett C. M., Nelson K. E. Science. 2006;312:1355–1359. doi: 10.1126/science.1124234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Macpherson A. J., Gatto D., Sainsbury E., Harriman G. R., Hengartner H., Zinkernagel R. M. Science. 2000;288:2222–2226. doi: 10.1126/science.288.5474.2222. [DOI] [PubMed] [Google Scholar]
- 42.Macpherson A. J., Uhr T. Science. 2004;303:1662–1665. doi: 10.1126/science.1091334. [DOI] [PubMed] [Google Scholar]
- 43.Gilmore M. S., Ferretti J. J. Science. 2003;299:1999–2002. doi: 10.1126/science.1083534. [DOI] [PubMed] [Google Scholar]
- 44.Clayton T. A., Lindon J. C., Cloarec O., Antti H., Charuel C., Hanton G., Provost J. P., Le Net J. L., Baker D., Walley R. J., et al. Nature. 2006;440:1073–1077. doi: 10.1038/nature04648. [DOI] [PubMed] [Google Scholar]
- 45.Cloarec O., Dumas M. E., Trygg J., Craig A., Barton R. H., Lindon J. C., Nicholson J. K., Holmes E. Anal. Chem. 2005;77:517–526. doi: 10.1021/ac048803i. [DOI] [PubMed] [Google Scholar]
- 46.Wold S., Antti H., Lindgren F., Ohman J. Chemometrics Intelligent Lab. Syst. 1998;44:175–185. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.