Abstract
The endosymbiotic bacteria in the genus Wolbachia are capable of inducing a wide range of reproductive abnormalities in their hosts, including cytoplasmic incompatibility (CI), which could lead to the replacement of uninfected host populations with infected ones. Because of this, Wolbachia have attracted considerable interest as a potential mechanism for spreading disease-blocking transgenes through vector populations. Here we report the establishment of double Wolbachia transinfection by direct adult microinjection of Wolbachia from naturally double-infected Aedes albopictus to Aedes aegypti, the most important mosquito vector of infectious viral diseases, and a mosquito in which natural Wolbachia infections are not known to occur. We further demonstrate that incomplete CI is induced in these double-transinfected mosquitoes. Comparisons of fitness traits between naturally uninfected and transinfected Ae. aegypti lines indicated one significant difference in favor of the latter, namely, an increased number of eggs laid. Levels of CI expression corresponded to the Wolbachia density. There were large differences in relative Wolbachia density between reproductive and nonreproductive tissues in both Ae. albopictus and transinfected Ae. aegypti, except Malpighian tubule, which implied the preferred establishment of Wolbachia within reproductive tissue. Results from a simulation model confirm that population replacement by transinfected Ae. aegypti is possible over time. The establishment of Wolbachia double infections in Ae. aegypti by direct adult microinjection and the demonstration of CI expression in this new host suggest that Wolbachia could be experimentally transferred into vector species and could also be used as a gene-driving system to genetically manipulate vector populations.
Keywords: microinjection, transfer, replacement, tissue tropism, cytoplasmic incompatibility
Because of the inefficiency of vaccines for curing many vector-borne diseases, the genetic modification of arthropod vectors has been seriously considered as a means to control these diseases (1–4). Stable gene transformations of mosquitoes have been reported, indicating the possibility to genetically modify vectors to control diseases (2–5). Most such strategies, however, require a high frequency of transgenic vectors to spread in natural populations to achieve population replacement (6). To ensure the spread of disease-blocking transgenes and to reduce the number of transgenic vectors needed for release into natural populations, integration with gene drivers such as Wolbachia may be necessary and should be considered.
Wolbachia are maternally inherited endosymbiotic bacteria recognized to infect a broad range of arthropod species and some filarial nematodes (7). Various surveys have estimated that 16–76% of insect species are infected with Wolbachia (8, 9) and that they are capable of inducing a wide range of reproductive abnormalities in their hosts, including cytoplasmic incompatibility (CI) (10). CI results in a failure of karyogamy, perhaps by delaying nuclear envelope breakdown and mitosis (11), and consequently may promote Wolbachia invasion of uninfected populations because infected females are able to mate and produce offspring successfully with both infected and uninfected males, whereas uninfected females are unable to produce offspring when they mate with infected males (10, 12). Theoretically, insects harboring CI-inducing Wolbachia would spread rapidly into uninfected populations, causing population replacement (12). The powerful spreading capability of Wolbachia via CI has attracted considerable attention as a potential gene-driving system (12, 13). The expression of transgenes could be straightforward if the population invasion ability of Wolbachia is used (12). However, within this theoretical framework, which still lacks empirical support, the capability of Wolbachia to invade and maintain themselves in host populations depends on three main parameters: (i) the strength of CI, (ii) maternal transmission efficiency, and (iii) fitness effects on the host (14).
Aedes aegypti, one of the most important mosquito vectors, is commonly recognized to cause dengue and yellow fever epidemics (15, 16). Wolbachia infection has never been detected in this particular species (12, 17). Therefore, the establishment of Wolbachia infection and induction of CI expression in Ae. aegypti would be an essential prerequisite to the possible application of Wolbachia as a transgene drive mechanism to aid in genetic control of this important vector species (13, 18). Syncytial embryo microinjection has been used extensively for Wolbachia transfer in various insect groups (18–21). Recently this technique was successfully used to introduce a single strain of Wolbachia from Aedes albopictus into Ae. aegypti (22). In this article we demonstrate the successful Wolbachia transfer and the establishment of double strains of Wolbachia from Ae. albopictus in Ae. aegypti via direct adult microinjection.
Results and Discussion
Establishment of Wolbachia-Superinfected Ae. aegypti.
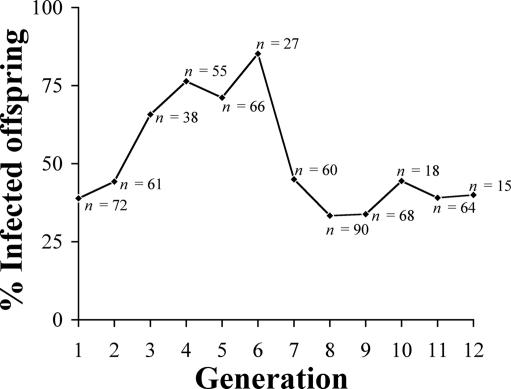
The Wolbachia extract was directly microinjected into 76 newly emerged adult females of naturally uninfected Ae. aegypti. Forty-nine percent of injected mosquitoes survived, and 93% of surviving adults tested positive for Wolbachia DNA by PCR, indicating infection with Wolbachia. Isofemale lines were established by using surviving mosquitoes that were PCR-positive for Wolbachia and were designated AegW. Each generation of AegW was monitored for the transmission efficiency of Wolbachia by using a specific wsp-based PCR assay (23), and infected offspring were chosen to start a new generation as shown in Fig. 1. Transmission efficiency of Wolbachia was low in G1 (39%, n = 72) but increased gradually (G2, 44%, n = 61; G3, 66%, n = 38; G4, 76%, n = 55; G5, 71%, n = 66) until it reached a maximum at G6 (85%, n = 27). The transmission efficiency thereafter decreased to 45% in G7 (n = 60) and was stably maintained at ≈40% until the last observation at G12 (G8, 33%, n = 90; G9, 34%, n = 68; G10, 44%, n = 18; G11, 39%, n = 64; G12, 40%, n = 15). Since then, the AegW colony routinely has been maintained in the insectary at 25–27°C and 70–75% relative humidity by selection of the isofemale lines with PCR-positive results, and recently up to 81.8% (G26, n = 11) of infection rate has been observed in some of the selected isofemale lines.
Fig. 1.
Transmission efficiency in different generations of transinfected Ae. aegypti populations. Only infected mosquitoes were used to produce the next generation.
To confirm the establishment of Wolbachia within AegW populations, wsp partial sequences were amplified and sequenced from multiple generations (G4, G6, G8, and G10). Both wAlbA and wAlbB strains were found in AegW. The sequences showed >99.2% identity to wAlbA and >98.4% identity to wAlbB, indicating that AegW were infected with Wolbachia derived from double-infected Ae. albopictus. The presence of Wolbachia in multiple generations of AegW shows that these bacteria are capable of being maternally transmitted in this novel host. The slight differences in DNA base changes within wsp sequences of AegW could be caused by PCR or sequencing (Taq) errors, stochastic cloning of relatively rare variants, or actual Wolbachia adaptation to the new host. However, the DNA base alteration occurred at the same sites and the same bases under different generations, whereas Wolbachia of transinfected Ae. albopictus that carried the same Wolbachia strains did not show DNA base alteration (100% identity; unpublished data). This finding implies that Wolbachia may have adapted to its new Ae. aegypti host, possibly through changes in wsp protein, which mediates between the host cell and the Wolbachia bacterium. However, we report here the successful establishment of double Wolbachia strains (wAlbA and wAlbB) in Ae. aegypti host by direct microinjection into an adult stage.
Syncytial embryonic microinjection is commonly used to establish Wolbachia infections in desirable insects because it allows Wolbachia placement within developing pole cells (and thus germ-line tissues) and yields high rates of establishment of successful transinfected lines. However, until recently high mortality rates in mosquitoes resulted in low transinfection frequencies relative to the starting number of embryos (22). A different microinjection approach, direct adult microinjection, has been taken in this work for establishing Wolbachia infection in Ae. aegypti. Unlike the earlier approach, it is much simpler and has several benefits, including no need for the expertise required for embryonic microinjection. A disadvantage of this approach is the low Wolbachia number caused by indirect establishment in germ cells in G0, which results in a high proportion of uninfected cytoblasts (11). This finding contrasts with embryonic microinjection in which syncytial eggs are directly infected with Wolbachia in the pole cell region, resulting in codevelopment of Wolbachia and the host’s embryo, and especially germ-line tissues, potentially leading to a higher number of Wolbachia within the reproductive tissues.
CI Expression in Transinfected Ae. aegypti.
To determine the capability for CI expression and the effect of multiple male and female matings on CI expression, test crosses were established between transinfected Ae. aegypti (AegW; G5 and G6) and naturally uninfected Ae. aegypti (Aeg) as shown in Table 1. Two crossing experiments were conducted. In the first experiment (Table 1, Exp. 1), comprising single-pair copulations with one male and one female, we found that crosses between AegW males and Aeg females (c) produced 50.68% egg hatch, which was significantly lower than that for naturally uninfected crosses [d, 87.53%; Mann–Whitney U test (U), P = 0.002] and is indicative of incomplete CI expression. In contrast, the other reciprocal cross (Aeg males and AegW females) did not give any significant reduction in the average hatch rate (a vs. b; U, P = 0.262). The occurrence of incomplete CI probably reflects the low Wolbachia numbers in G5 and G6 of AegW used in the experiments (19). To clarify any effect of multiple mating on the net egg hatchability, test crosses were conducted with swarming females and males to allow freedom of mate choice and number of matings (Table 1, Exp. 2). It was found that expected incompatible crosses (g) produced 50.94% egg hatch, which was significantly lower than that of uninfected crosses (h, 89.94%; U, P < 0.001), and the egg hatch rate in reciprocal crosses (e vs. f; U, P = 0.623) was not significantly lower. Nor was there any significant difference in offspring production between these two crosses, thus indicating that multiple matings, if they occurred, and free mate choice did not cause a negative effect on CI.
Table 1.
CI expression, as shown by the hatch rate of eggs produced, in crosses between Ae. aegypti transinfected with Wolbachia from double-infected Ae. albopictus and naturally uninfected Ae. aegypti
| Cross (female × male) | Mean % egg hatch ± SE | Total no. of eggs counted | No. of crosses | Comparison | P value (U) |
|---|---|---|---|---|---|
| Exp. 1 | |||||
| AegW × AegW, a | 78.79 ± 6.77 | 785 | 12 | ||
| AegW × Aeg, b | 89.19 ± 2.47 | 1,007 | 15 | b, a | 0.262 |
| Aeg × AegW, c | 50.68 ± 8.65 | 1,092 | 22 | c, d | 0.002 |
| Aeg × Aeg, d | 87.53 ± 5.15 | 1,076 | 15 | ||
| Exp. 2 | |||||
| AegW × AegW, e | 87.88 ± 1.71 | 1,962 | 36 | ||
| AegW × Aeg, f | 85.36 ± 3.18 | 1,934 | 37 | f, e | 0.623 |
| Aeg × AegW, g | 50.94 ± 5.36 | 1,720 | 37 | g, h | <0.001 |
| Aeg × Aeg, h | 89.04 ± 1.17 | 1,936 | 38 |
Two crossing experiments (Exp.) were conducted: Exp. 1 used single-pair copulations; in Exp. 2, males and females were allowed to swarm and thus were free to choose mates and number of copulations. Crosses were conducted under controlled conditions (27°C, 72% relative humidity, and 10% sucrose) for 3 days before females were allowed to blood-feed and were individually isolated into vials filled with egg-collecting paper. The paper was removed and dried after 3 days. The eggs were counted and incubated for 24 h in deoxygenated water, after which hatched offspring were counted. All females were checked for insemination by examination of spermathecae for sperm. Unmated females were excluded from analysis. In the single-pair cross, only crosses in which AegW males were shown to be PCR-positive were included.
Fitness of Transinfected Ae. aegypti.
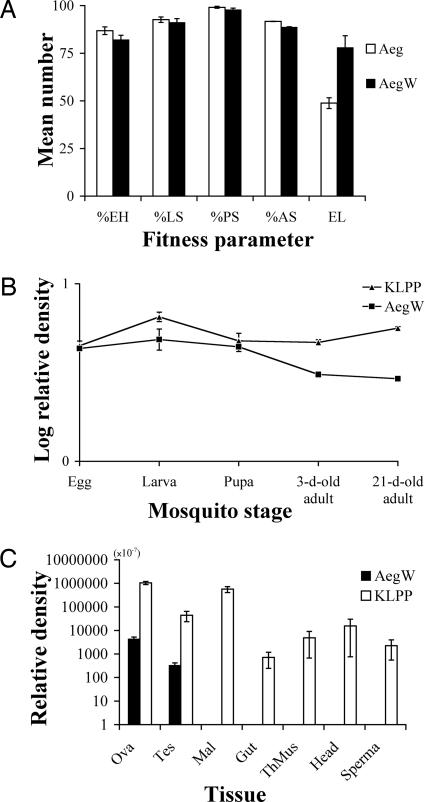
The fitness of AegW (G6, n = 28) as measured by percentage of egg hatch, percentages of survival of larvae, pupae, and adults, and number of eggs laid was compared with that of Aeg (n = 28). Differences were not significant for percentage of egg hatch (AegW = 82.1 ± 2.3 and Aeg = 86.8 ± 2.0; U, P = 0.11), percentage of larval survival (91.2 ± 2.0 and 92.6 ± 1.4; U, P = 0.74), percentage of pupal survival (97.8 ± 0.9 and 99.1 ± 0.5; U, P = 0.09), and percentage of adult survival (88.7 ± 0.2 and 91.7 ± 0.1; U, P = 0.902), whereas a significant difference was found for the number of eggs laid (78.0 ± 6.2 and 48.8 ± 2.8; t = 4.276, df = 37.65, P < 0.001) (Fig. 2A). Transgenic mosquitoes produced fewer eggs in the study by Irvin et al. (24). However, in this study we found a high fecundity of transinfected AegW females. In addition, the ratio of male-to-female offspring did not significantly deviate from 1:1, and no significant differences were found between offspring sex ratios [AegW (n = 28) = 1.2 ± 0.1 and Aeg (n = 28) = 1.2 ± 0.2; U, P = 0.555]. The equality of sex ratio and the higher fecundity encourage CI expression by two beneficial outcomes: infected females increase infected offspring, whereas infected males decrease uninfected offspring in the next generations. These phenomena distort the ratio of infected to uninfected individuals in successive generations. Because our fitness determination was for the first gonotrophic cycle only, additional observations of fitness in multiple generations and gonotrophic cycles are important to be sure of beneficial effects of Wolbachia on AegW fitness.
Fig. 2.
Fitness and levels of relative Wolbachia density. (A) Fitness of AegW (G6) and Aeg, i.e., mean percentage of egg hatch (EH), percentage of larval survival (LS), percentage of pupal survival (PS), percentage of adult survival (AS), and number of eggs laid (EL). (B) Log relative Wolbachia density at different developmental stages in AegW and KLPP. (C) Relative Wolbachia density in different tissues in AegW and KLPP. The error bars represent standard errors.
Effect of Wolbachia Density on CI Expression of Transinfected Ae. aegypti.
Lower CI expression in transinfected lines has been observed in many studies (19, 21) and suggests that CI expression depends on host factors, Wolbachia adaptation, or a threshold level for Wolbachia densities. However, the successful transfer of the same Wolbachia strains to tetracycline-treated Drosophila simulans, the ability of the Wolbachia strains to induce CI, and their cytoskeleton associations implied Wolbachia adaptation to the new host (18). To investigate the correlation of Wolbachia density with CI expression, the densities of Wolbachia were measured by using quantitative real-time PCR. Comparison of internal host gene control between AegW and KLPP was conducted, and it was found that host copy numbers of AegW were not significantly different from KLPP [AegW = (3.2 ± 0.3) × 109 and KLPP = (2.5 ± 0.3) × 109; t = 1.832, df = 38, P = 0.075] (Table 2); therefore, AegW and KLPP have approximately the same cell numbers under the control condition/environment. The correlation between relative Wolbachia density in adults and the degree of CI expression in AegW and KLPP mosquitoes is shown in Table 2. Transinfected AegW (n = 33) were found to have Wolbachia density 165 times lower than that of the naturally double-infected Ae. albopictus (KLPP, n = 25) (t = 6.304, df = 24, P < 0.001). This finding corresponded to the level of CI expression in the two mosquitoes, in which the percentage of unhatched eggs in the nonpermissible crosses in Ae. aegypti (Aeg female × AegW male; n = 59; 49.16 ± 4.61) was significantly lower than that of the corresponding crosses in Ae. albopictus [uninfected female (KLPPT) × KLPP male, n = 58; 100 ± 0.0] (U, P < 0.001). Low Wolbachia density could result in a proportion of uninfected cytoblasts as a result of stochastic loss of Wolbachia during mitosis and the production of spermatids containing amounts of Wolbachia insufficient to elicit CI (19), which may explain the incomplete CI of AegW (G5 and G6). To determine the influence of host factors on AegW, relative Wolbachia density was observed in different stages of AegW and KLPP. The relative Wolbachia density of KLPP constantly remained at the same level in all developmental stages (Fig. 2B), whereas AegW did not change at the beginning stages (egg, larva, and pupa stages) but started to decrease when it entered the 3-day-old adult stage. No significant differences in Wolbachia densities were detected at the egg stage (t test, t = 0.5, df = 7, P = 0.632), the larval stage (t = 1.959, df = 15.331, P = 0.069), and the pupal stage (t = 0.548, df = 6, P = 0.603). The lack of differences in Wolbachia density in the eggs and the immature stages between AegW and KLPP indicates that host factors may not be important during these stages. However, relative Wolbachia density of AegW was significantly lower in the 3-day-old adults (t = 8.064, df = 19, P < 0.001) and also in the 21-day-old adults (t = 20.023, df = 22, P < 0.001) than in those of KLPP. The possibility that host factors may suppress an increase of Wolbachia in AegW is not conclusive and requires further investigation. wMelPop in D. simulans was found to have a Wolbachia density similar to wMelPop in Drosophila melanogaster after wMelPop was introduced into D. simulans (18). D. simulans, therefore, appears to have no repressing factors, and Wolbachia may easily establish without host interference (18, 25). One possible way to prove that differences in Wolbachia densities are likely due to host factors could be to reintroduce Wolbachia back into uninfected Ae. albopictus and determine Wolbachia density.
Table 2.
Levels of relative Wolbachia density of the host cell numbers and levels of CI expression measured by the mean percentage of unhatched eggs in expected CI crosses in transinfected Ae. aegypti (AegW, recipient) and double-infected Ae. albopictus (KLPP, donor)
| % of unhatched eggs | Wolbachia density, × 10−3 | Host cell no., × 109 | |
|---|---|---|---|
| KLPP donor | 100 ± 0.0 (58) | 3.3 ± 0.6 (25) | 2.5 ± 0.3 (20) |
| AegW recipient | 49.16 ± 4.61 (59) | 0.02 ± 0.003 (33) | 3.2 ± 0.3 (20) |
The numbers of KLPP donors and AegW recipients are listed in parentheses.
Relative Wolbachia density was determined in different tissues, including ovaries, testes, Malpighian tubule, midgut, thoracic muscles, head, and spermatheca to demonstrate tissue tropism of AegW and KLPP. Wolbachia was detected in all tissues of KLPP, whereas infection was found only in the ovaries and testes of AegW. There were differences in Wolbachia density between AegW and KLPP: ovaries, AegW = (4.4 ± 0.9) × 10−4 (n = 20), and KLPP = (1.0 ± 0.2) × 10−1 (n = 12); testes, (3.3 ± 0.9) × 10−5 (n = 10) and (4.4 ± 2.1) × 10−3 (n = 6); Malpighian tubule, 0.0 ± 0.0 (n = 12) and (5.7 ± 1.6) × 10−2 (n = 9); midgut, 0.0 ± 0.0 (n = 12) and (7.1 ± 4.7) × 10−5 (n = 12); thoracic muscles, 0.0 ± 0.0 (n = 12) and (4.9 ± 4.2) × 10−4 (n = 12); head, 0.0 ± 0.0 (n = 12) and (1.6 ± 1.5) × 10−3 (n = 12); spermatheca, 0.0 ± 0.0 (n = 12) and (2.3 ± 1.7) × 10−4 (n = 9) (Fig. 2C). The nondetection of Wolbachia in nonreproductive tissues of AegW does not definitely indicate no infection. AegW could display different tissue tropism in different host species, or it is possible that the nonreproductive tissues of AegW harbor Wolbachia densities under the minimum detection level of quantitative PCR. Differences in relative Wolbachia density were observed among different tissues of KLPP, with the average Wolbachia density of nonreproductive tissues being comparatively lower than that for reproductive tissues except in Mulpighian tubules. However, the highest average density of Wolbachia in AegW and KLPP was found in the reproductive tissues. The large differences in average Wolbachia density between reproductive and nonreproductive tissues in both AegW and KLPP could imply that Wolbachia prefers reproductive tissues over nonreproductive tissues.
Transinfected Ae. aegypti as a Potential Tool for Vector Population Replacement.
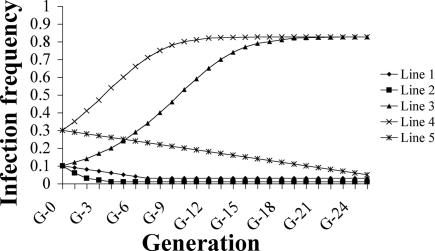
To predict population replacement by AegW, the Dobson model was chosen and applied with our parameters as shown in Fig. 3. The Dobson model is specifically modified to study population replacement of uninfected Ae. albopictus by double-infected Ae. albopictus and could describe infections affording a host fecundity advantage (26). By using 10% initial AegW and the highest transmission efficiency observed in G6 (85%), infection frequency exceeds 82.6% by generation 23 and remains fixed at 82.6% (Fig. 3, line 3). One hundred percent infection frequency cannot be reached because the chosen 85% transmission efficiency of AegW results in 15% uninfected progeny in the next generation. If the initial AegW is increased to 30%, infection frequency can exceed 82.6% by G16 (Fig. 3, line 4). However, the transmission efficiency of AegW was ≈40% after G6 (Fig. 1), which, in the simulation model, would predict a decrease in infection frequency to nearly 0 within five generations (Fig. 3, line 2). All three previous predictions were based on higher fecundity of AegW. However, Xi et al. (22) found that their transinfected lines had equal fecundity to control lines, and including this in the simulation indicates that replacement does not occur for both 10% and 30% initial AegW under equal fecundity and that AegW frequency decreases until it remains fixed at nearly 0 (Fig. 3, lines 1 and 5). So, according to our simulations, AegW will invade and replace uninfected populations if AegW has higher fecundity at 85% transmission efficiency and 51% relative hatch rate starting with at least 10% initial AegW. The prediction that AegW will not completely replace an uninfected population is in contrast to that of Xi et al. (22), but it does provide important information that there is a potential to use Wolbachia as a gene driver in vector populations. Incomplete CI of AegW is a major barrier in promoting the spread of AegW in uninfected populations. However, Xi et al. (22) were able to demonstrate complete CI and the invasion and establishment of CI-inducing Wolbachia in a laboratory population of this mosquito. A recent increase in the infection rate of AegW up to 81.8% in G26 indicates the possibility that this transinfected colony could reach high Wolbachia density, high maternal transmission, and complete CI in the higher generations, which requires further investigation.
Fig. 3.
Simulated changes in Wolbachia infection frequency. All simulations were generated by using the Dobson-specific Ae. albopictus model (26). Fecundity effect by Wolbachia, transmission efficiency, and initial infection rate was as follows for each line: line 1, 1.0, 0.85, and 0.1; line 2, 0.71, 0.4, and 0.1; line 3, 0.71, 0.85, and 0.1; line 4, 0.71, 0.85, and 0.3; line 5, 1, 0.85, and 0.3. Relative hatch rate was 0.51.
In this work, double infections of Wolbachia (wAlbA and wAlbB strains) were established and caused incomplete CI at G5 and G6 of transinfected Ae. aegypti. These results were different from the work of Xi et al. (22), whose single infection, wAlbB, was established and caused complete CI, although the same Wolbachia strains were transferred into the same host species. The lower infection rate of wAlbA relative to wAlbB is used to explain the establishment of a single infection in Xi et al. (22). Concentrating Wolbachia numbers during extraction before direct microinjection may be a key to the establishment of double infections because the numbers of wAlbA are lower than wAlbB in Ae. albopictus (27). In addition, the lumen space of the adult for loading Wolbachia is bigger than the egg; therefore, more Wolbachia numbers can be injected into the adult, providing higher potential of establishment of double infections than the egg. The random establishment of Wolbachia in different tissues during G0 would result in low infection of Wolbachia in reproductive tissues and thus incomplete transmission of Wolbachia in the early generation. In contrast, Xi et al. (22) used syncytial embryonic microinjection, which would result in codevelopment of Wolbachia and pole cells forming the germ-line tissues and thus a higher establishment of Wolbachia within reproductive tissues. However, alone this is unlikely to explain the incomplete CI expression observed in our work compared with the complete CI expression reported by Xi et al. (22) because from G1 onward the transinfected Wolbachia reside within the syncytial egg in both cases and can replicate to the controlled density in each developing tissue type. Thus, the possibility of some additional host factors suppressing the replication of Wolbachia within our AegW lines, but not Xi et al. (22), remains to be clarified.
This study clearly shows that crosses of AegW males with uninfected Ae. aegypti females result in significantly lower hatch rate than other crosses and that CI expression is not affected by multiple matings. The level of CI expression corresponds with Wolbachia copy number within the AegW host. To develop optimum usage of Wolbachia as a gene driver, the isofemale lines of Wolbachia-double-infected AegW have been repeatedly selected for each generation to increase the level of maternal transmission and the number of Wolbachia, which will probably, then, lead to high levels of CI expression. This study demonstrates that there is a potential for using Wolbachia as a gene-driving system. By integrating Wolbachia with transgenic mosquitoes (2–5) it may be possible to manipulate vector populations genetically (13, 18). Wolbachia is not the only choice for driving genes into vector populations (12, 28), but this study demonstrates that it is one possible option.
Materials and Methods
Wolbachia Detection and DNA Analysis.
DNA was extracted from individual eggs, larvae, pupae, adults, or tissues by using the Holmes–Bonner method (29). PCR was performed as described (23). PCR products were diluted 500-fold and reamplified by using the previous profile (23) except for the modified 52°C annealing temperature and internal primers (151F, 5′-TGG TTA CAA AAT GGA CGA CA; 599R, 5′-CAC CAA CAG TGC TGT AAA GAA C) to increase sensitivity and specificity. Mitochondrial 12S rDNA primers were used as a quality control for DNA extraction (30). PCR products were resolved by 1% (wt/vol) agarose gel electrophoresis, stained with ethidium bromide, and visualized under UV light. Desirable products were ligated into pGEM-T vectors (Promega, Madison, WI). At least three clones were purified and sequenced on an automated sequencer (Applied Biosystems, Foster City, CA). The wsp sequences were aligned by using a cluster algorithm followed by manual modification based on amino acid translation (DNASTAR, Madison, WI).
Wolbachia Microinjection.
Wolbachia were crudely purified from ovaries of 2-week-old Ae. albopictus double-infected with wAlbA and wAlbB strains. The ovaries were dissected under PBS and homogenized in homogenizing buffer (31). The homogenate was filtered through a 0.95-μm pore size and centrifuged at 200 × g for 5 min to remove cellular debris. Then the suspension containing Wolbachia was pelleted at 4,000 × g for 10 min. The pellet was resuspended in homogenizing buffer. Twenty-four-hour-old Ae. aegypti recipients were knocked down and microinjected directly in the region between the posterior pronotum and the sternopleuron by glass needles with manually cut tips. Virgin females were mated to uninfected males the next day to establish isofemale lines.
Quantitative Real-Time PCR.
DNA was extracted as previously described. A real-time quantitative PCR assay based on a single-copy gene (wsp) encoding a surface protein of Wolbachia was used to determine Wolbachia copy number in the hosts. Primers were designed specifically to detect wAlbA (GF and AR) and wAlbB (GF and BR) strains and amplified 124- to 250-bp regions of the wsp gene (GF, 5′-GGT TTT GCT GGT CAA GTA A; AR, 5′-GCA TCT TTG GTA ACT ACT TTT; BR, 5′-GCT GTA AAG AAC GTT GAT C). A specific TaqMan probe (5′-FAM TGT TAG TTA TGA TGT AAC TCC AGA A-TAMRA) for the central region of the PCR product was designed and used to measure the amount of Wolbachia. PCR was performed under the following conditions: 15 min at 95°C, then 45 cycles of 94°C for 1 min, 50°C for 1 min, and 60°C for 1 min. The PCR included a Probe PCR Master Mix (Qiagen, Hilden, Germany), 0.4 μM concentration of each primer, 0.2 μM TaqMan probe, and 2 μl of template DNA made up to a 25-μl total volume. A standard curve was constructed by using wAlbA and wAlbB amplicons that had been previously cloned into pGEM-T vector (Promega), linearized with NcoI, and quantified as template. Three replicates were performed and averaged for each sample. Strain-specific primers to wAlbA and wAlbB were applied to all samples, and then total Wolbachia copy number was calculated by integrating both numbers. Host cell number was measured based on a single-copy gene (defensin) encoding an insect immunity. Primers were designed specifically to defensin D of Ae. albopictus and defensin A of Ae. aegypti (Def-F, 5′-ATC ACT GGT GCT TAC CCA CAG G; Def-R2, 5′-GAC GCA CAC CTT CTT GGA GTT G). SYBR Green was used to measure the amount of host cell number under the following conditions: 15 min at 95°C, then 45 cycles of 94°C for 1 min, 50°C for 1 min, and 72°C for 1 min. The PCR included SYBR Green PCR Master Mix (Applied Biosystems), 0.5 μM concentration of each primer, and 2 μl of template DNA made up to a 25-μl total volume. A standard curve was constructed as described above. Three replicates were performed and averaged for each sample.
Acknowledgments
We thank R. D. J. Butcher and J. R. Milne for editing and reviewing the manuscript and for helpful comments; S. L. O’Neill and E. A. McGraw for advice on microinjection techniques; R. Khumthong for primer design; and P. Olanratmanee, N. Klinpikul, S. Theinthong, and K. Theinthong for technical assistance. This work was supported by Mahidol University Research Grant SCBI-47-T-217, Thailand Research Fund Grants RGJ/PHD/0079/2542 and RDG4530034, and Thailand Research Fund/National Center for Genetic Engineering and Biotechnology Special Program for Biodiversity Research and Training Grant BRT R245008.
Glossary
Abbreviations
- CI
cytoplasmic incompatibility
- U
Mann–Whitney U test.
Footnotes
Conflict of interest statement: No conflicts declared.
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Beaty B. J. Proc. Natl. Acad. Sci. USA. 2000;97:10295–10297. doi: 10.1073/pnas.97.19.10295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Catteruccia F., Noland T., Loukeris T. G., Blass C., Savakis C., Kafatos F. C., Crisanti A. Nature. 2000;405:959–962. doi: 10.1038/35016096. [DOI] [PubMed] [Google Scholar]
- 3.Coates C. J. Nature. 2000;405:900–901. doi: 10.1038/35016192. [DOI] [PubMed] [Google Scholar]
- 4.Ito J., Ghosh A., Moreira L., Wimmer E. A., Jacobs-Lorena M. Nature. 2002;417:452–455. doi: 10.1038/417452a. [DOI] [PubMed] [Google Scholar]
- 5.Coates C. J., Jasinskiene N., Miyashiro L., James A. A. Proc. Natl. Acad. Sci. USA. 1998;95:3748–3751. doi: 10.1073/pnas.95.7.3748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.James A. A. In: Insect Transgenesis: Methods and Applications. Handler A. M., James A. A., editors. Boca Raton, FL: CRC; 2002. pp. 319–333. [Google Scholar]
- 7.Lo N., Casiraghi M., Salati E., Bazzocchi C., Bandi C. Mol. Biol. Evol. 2002;19:341–346. doi: 10.1093/oxfordjournals.molbev.a004087. [DOI] [PubMed] [Google Scholar]
- 8.Werren J. H., Windsor D., Guo L. R. Proc. R. Soc. London Ser. B; 1995. pp. 197–204. [Google Scholar]
- 9.Jeyaprakash A., Hoy M. A. Insect Mol. Biol. 2000;9:393–405. doi: 10.1046/j.1365-2583.2000.00203.x. [DOI] [PubMed] [Google Scholar]
- 10.O’Neill S. L., Hoffmann A. A., Werren J. H. Influential Passengers: Inherited Microorganisms and Arthropod Reproduction. Oxford: Oxford Univ. Press; 1997. [Google Scholar]
- 11.Tram U., Sullivan W. Science. 2002;296:1124–1126. doi: 10.1126/science.1070536. [DOI] [PubMed] [Google Scholar]
- 12.Sinkins S. P. Insect Biochem. Mol. Biol. 2004;34:723–729. doi: 10.1016/j.ibmb.2004.03.025. [DOI] [PubMed] [Google Scholar]
- 13.Turelli M., Hoffmann A. A. Nature. 1991;353:440–442. doi: 10.1038/353440a0. [DOI] [PubMed] [Google Scholar]
- 14.Turelli M., Hoffmann A. A. Genetics. 1995;140:1319–1338. doi: 10.1093/genetics/140.4.1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gubler D. J. In: The Arboviruses: Epidemiology and Ecology. Monath T. P., editor. Vol. II. Boca Raton, FL: CRC; 1989. pp. 223–260. [Google Scholar]
- 16.Monath T. P. Proc. Natl. Acad. Sci. USA. 1994;91:2395–2400. doi: 10.1073/pnas.91.7.2395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kittayapong P., Baisley K. J., Baimai V., O’Neill S. L. J. Med. Entomol. 2000;37:340–345. doi: 10.1093/jmedent/37.3.340. [DOI] [PubMed] [Google Scholar]
- 18.Braig H. R., Guzman H., Tesh R. B., O’Neill S. L. Nature. 1994;367:453–455. doi: 10.1038/367453a0. [DOI] [PubMed] [Google Scholar]
- 19.Boyle L., O’Neill S. L., Robertson H. M., Karr T. L. Science. 1993;260:1796–1799. doi: 10.1126/science.8511587. [DOI] [PubMed] [Google Scholar]
- 20.Kang L., Ma X., Cai L., Liao S., Sun L., Zhu H., Chen X., Shen D., Zhao S., Li C. Heredity. 2003;90:71–76. doi: 10.1038/sj.hdy.6800180. [DOI] [PubMed] [Google Scholar]
- 21.Sasaki T., Ishikawa H. Heredity. 2000;85:130–135. doi: 10.1046/j.1365-2540.2000.00734.x. [DOI] [PubMed] [Google Scholar]
- 22.Xi Z., Khoo C. C. H., Dobson S. L. Science. 2005;310:326–328. doi: 10.1126/science.1117607. [DOI] [PubMed] [Google Scholar]
- 23.Zhou W., Rousset F., O’Neill S. L. Proc. R. Soc. London Ser. B; 1998. pp. 509–515. [Google Scholar]
- 24.Irvin N., Hoddle M. S., O’Brochta D. A., Carey B., Atkinson P. W. Proc. Natl. Acad. Sci. USA. 2004;101:891–896. doi: 10.1073/pnas.0305511101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McGraw E. A., Merritt D. J., Droller J. N., O’Neill S. L. Proc. Natl. Acad. Sci. USA. 2002;99:2918–2923. doi: 10.1073/pnas.052466499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dobson S. L., Marsland E. J., Rattanadechakul W. Genetics. 2002;160:1087–1094. doi: 10.1093/genetics/160.3.1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dutton T. J., Sinkins S. P. Insect Mol. Biol. 2004;13:317–322. doi: 10.1111/j.0962-1075.2004.00490.x. [DOI] [PubMed] [Google Scholar]
- 28.Enserink M. Science. 2000;290:440–441. doi: 10.1126/science.290.5491.440. [DOI] [PubMed] [Google Scholar]
- 29.Holmes D. S., Bonner J. Biochemistry. 1973;12:2230–2338. doi: 10.1021/bi00736a023. [DOI] [PubMed] [Google Scholar]
- 30.O’Neill S. L., Giordano R., Colbert A. M. E., Karr T. L., Robertson H. M. Proc. Natl. Acad. Sci. USA. 1992;89:2699–2702. doi: 10.1073/pnas.89.7.2699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Braig H. R., Zhou W., Dobson S. L., O’Neill S. L. J. Bacteriol. 1998;180:2373–2378. doi: 10.1128/jb.180.9.2373-2378.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]